Average Atomic Mass Problems With Answers Sep 19 2017 0183 32 For a single atom this is the mass number but for an element it is the average atomic mass The easiest way to find the atomic mass is to look it up on a periodic table The atomic mass for each element is given in atomic mass units or grams per mole of atoms
Practice Problems Atomic Mass Answer Key The element bromine has three naturally occurring isotopes A mass spectrum of molecular Br 2 shows three peaks with mass numbers of 158 u 160 u and 162 u Use this information to determine which isotopes of Br occur in nature 79 u 81 u 169 2023 Google LLC In this video learn how to calculate average atomic mass using data how to determine the most abundant isotope and using hypothetical data to determine th
Average Atomic Mass Problems With Answers
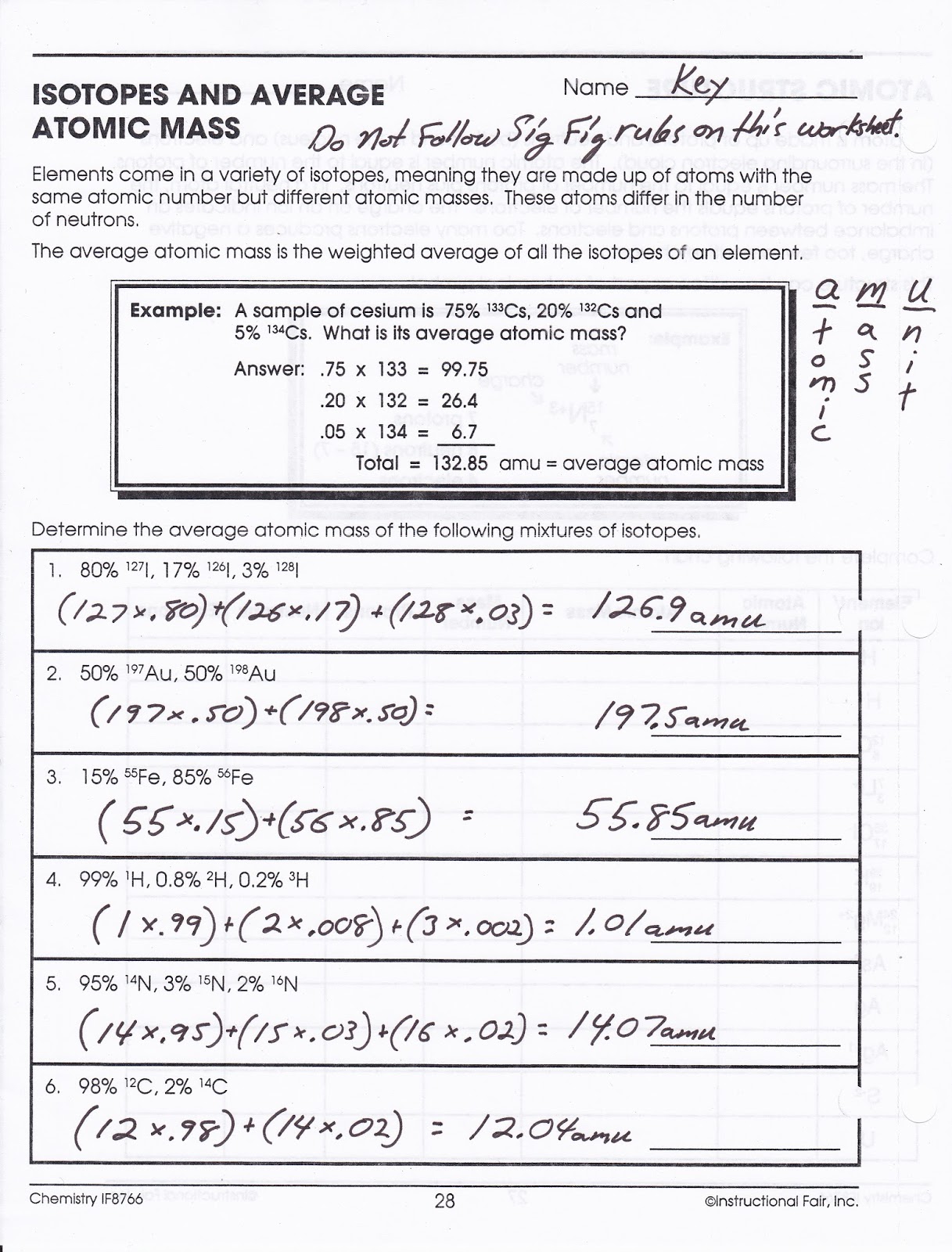 Average Atomic Mass Problems With Answers
Average Atomic Mass Problems With Answers
http://www.unmisravle.com/wp-content/uploads/2018/08/average_atomic_mass_worksheet_answers_with_work_4989965_0.jpg
The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element For example the atomic mass of carbon is reported as 12 011 amu atomic mass units Carbon is composed primarily of two isotopes carbon 12 and carbon 14
Templates are pre-designed documents or files that can be used for different purposes. They can save effort and time by providing a ready-made format and layout for creating various type of material. Templates can be utilized for individual or professional tasks, such as resumes, invites, flyers, newsletters, reports, presentations, and more.
Average Atomic Mass Problems With Answers

Average Atomic Mass And Percent Abundance Worksheet 2 And KEY Isotope

Average Atomic Mass Practice
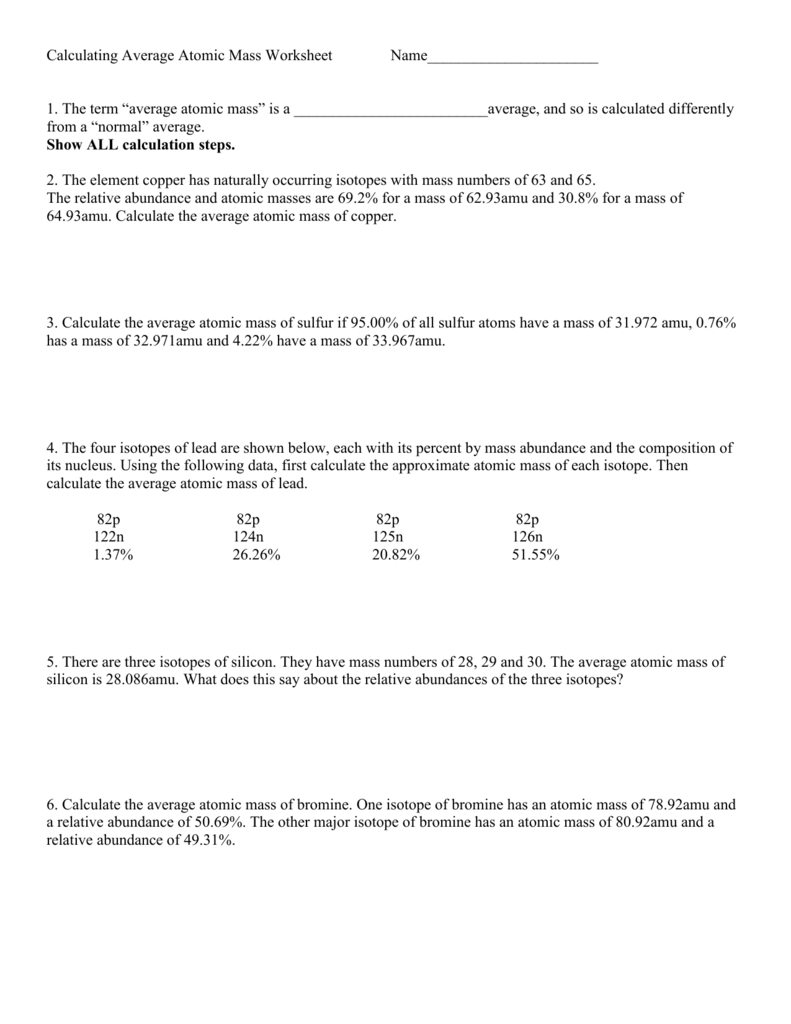
Calculating Average Atomic Mass Worksheet Name

3 Clear And Easy Ways To Calculate Atomic Mass WikiHow

Average Atomic Mass Problems

6 Average Atomic Mass Worksheet Answer Key FabTemplatez

https://study.com/skill/practice/calculating
Practice Calculating Average Atomic Mass with practice problems and explanations Get instant feedback extra help and step by step explanations Boost your Chemistry grade with Calculating
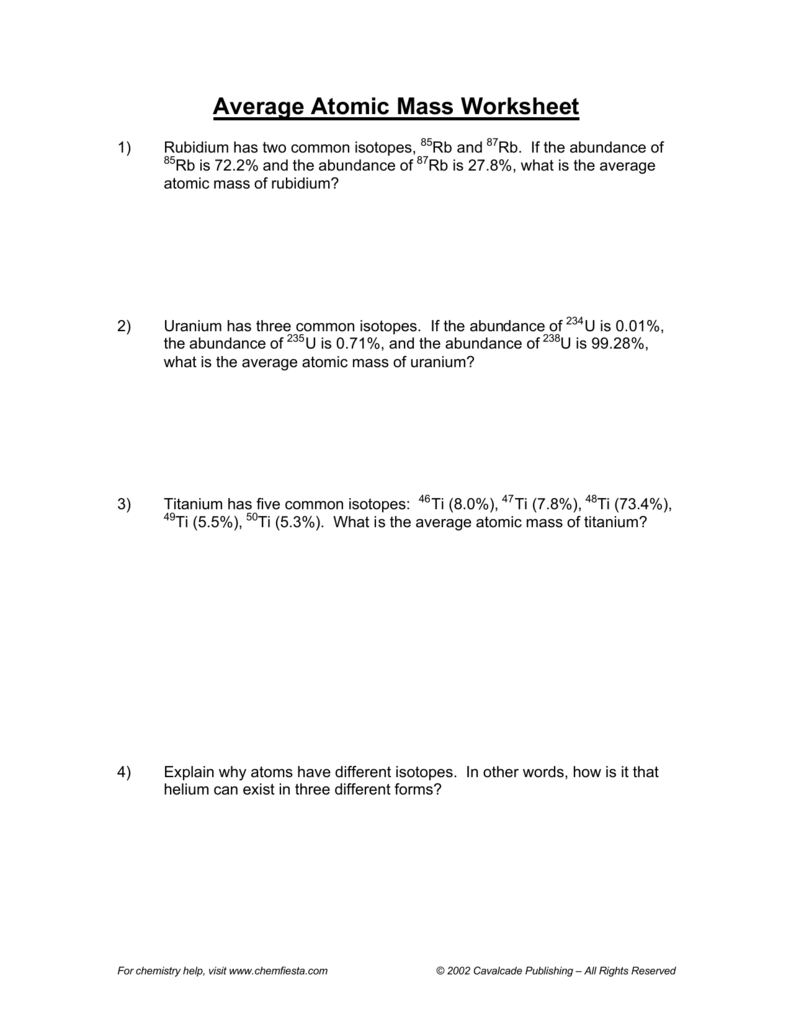
https://www.quizizz.com/admin/quiz/5c93b0ba02d7f
Average Atomic Mass Practice Problems quiz for 9th grade students Find other quizzes for Chemistry and more on Quizizz for free

https://chem.libretexts.org/Bookshelves/
Sep 20 2022 0183 32 Solution Step 1 List the known and unknown quantities and plan the problem Known Chlorine 35 atomic mass 34 969 text amu and percent abundance 75 77 Chlorine 37 atomic mass 36 966 text amu and percent abundance 24 23 Unknown Average atomic mass of chlorine

https://www.youtube.com/watch?v=rgixSP7PxS0
This chemistry video tutorial shows you how to calculate the average atomic mass of 2 or 3 isotopes It provides the equation formula for you to do so I

https://www.dentonisd.org//avg_atomic_mass_ws.pdf
1 Chlorine has two isotopes Chlorine 35 has an actual mass of 34 9689 u and chlorine 37 has a mass of 36 9659 u In any sample of chlorine atoms 75 771 will be chlorine 35 and 24 229 will be chlorine 37 Calculate the average
May 28 2020 0183 32 An element has the following natural abundances and isotopic masses 90 92 abundance with 19 99 amu 0 26 abundance with 20 99 amu and 8 82 abundance with 21 99 amu Calculate the average atomic mass of this element Answer 20 16 amu Click here to see a video of the solution Sep 25 2020 0183 32 Answer Exercise 4 7 1 1 4 7 1 1 The element strontium has the following natural abundances Calculate the average atomic mass 82 58 is 88 Sr with mass of 87 9056 amu 9 86 is 86 Sr with mass of 85 9093 amu 7 00 is 87 Sr with mass of 86 9089 amu 0 56 is 84 Sr with mass of 83 9134 amu Answer
Select your preferences below and click Start to give it a try Practice calculating the average atomic mass of fictional elements from their isotope masses and abundances with this online quiz