Bronsted Lowry Acids And Bases Worksheet Answers According to Bronsted Lowry theory an acid is a proton H donor and a base is a proton acceptor Ho H Example HCI OH Cl H O The HCI
Identify the Bronsted Lowry acid loses an H ion base gains an H ion conjugate acid and the conjugate base in each of the following The conjugate acid can donate a proton to the conjugate base to reform the original reactants in the reverse reaction HF H O H O F c acid c base acid
Bronsted Lowry Acids And Bases Worksheet Answers
 Bronsted Lowry Acids And Bases Worksheet Answers
Bronsted Lowry Acids And Bases Worksheet Answers
https://www.coursehero.com/thumb/0f/f7/0ff7a920fb8f00e287395e680d2e2b6c637c439c_180.jpg
SHORT ANSWER Answer the following questions in the space provided 1 Name the following compounds as acids sulfuric acid a H2SO4 sulfurous acid b H2SO3
Pre-crafted templates offer a time-saving solution for producing a varied series of files and files. These pre-designed formats and layouts can be used for different personal and expert jobs, including resumes, invitations, leaflets, newsletters, reports, presentations, and more, improving the content creation procedure.
Bronsted Lowry Acids And Bases Worksheet Answers

Defining Acids & Bases Worksheet by Chem Queen | TPT

bronsted lowry acids bases KEY - BRONSTED-LOWRY Name \ ACIDS AND BASES According to BronstedLowry theory an acid is a proton Ht donor and a base is | Course Hero

Bronsted Lowry Acids And Bases Worksheet - Fill and Sign Printable Template Online

SOLUTION: Acids and bases worksheet - Studypool
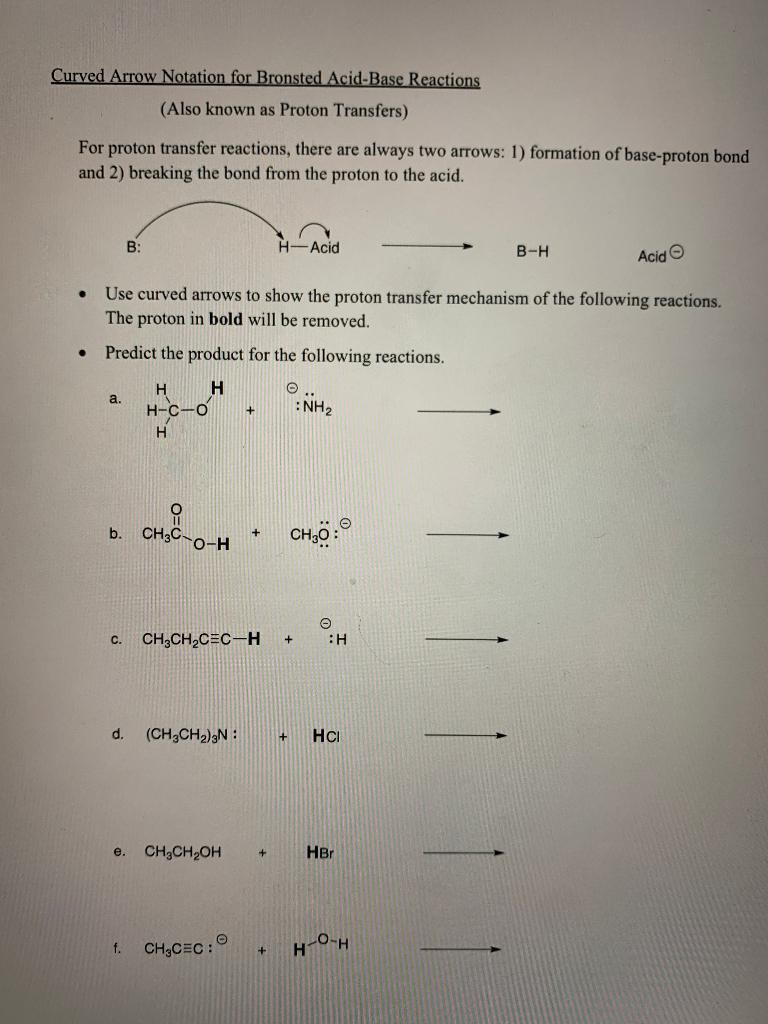
Solved Week 2 - Bronsted Acid Base Worksheet Introduction to | Chegg.com

Acid and Base Worksheet 1-07-08 Ans Key | PDF | Acid | Chemical Elements

https://web1.tvusd.k12.ca.us/gohs/myoung/Chem.%2013-14/Bronsted-Lowry%20Acid-Base%20Wks.%20Answers.pdf
In the exercise Bronsted Lowry Acids and Bases it was shown that after an acid has given up its proton it is capable of getting back that proton and

http://msschmidly.weebly.com/uploads/2/6/2/0/26201624/bronsted-lowry_acids_and_bases___answers.pdf
Worksheet Bronsted Lowry Acids and Bases Name Period Date Identity the Write the formulas for the conjugate bases formed by each of the following acids

https://gbutler.edublogs.org/files/2016/01/Bronsted-Lowry-Acids-Bases-Worksheet-Solutions-1viv9ky.pdf
3 Write balanced equations showing the reaction of the following weak acids with water 8 marks a H2C6H6O6 b C6H5OH c CH3COOH d HCO3

https://www.plps-k12.org/site/handlers/filedownload.ashx?moduleinstanceid=2482&dataid=3047&FileName=Ch%2014%20Conj%20Acid-Base%20Salts%20WS%20-%20Key.pdf
In the exercise Bronsted Lowry Acids and Bases it was shown that after an acid has given up its proton it is capable of getting back that proton and acting

https://www.coursehero.com/file/22954077/Worksheet-Bronsted-Lowry-Acids-and-Bases/
Worksheet Bronsted Lowry Acids and Bases Name Period Date Identity the conjugation acid base pairs in the following reactions An acid donates a proton to
In each reaction identify the Br nsted Lowry acid the Br nsted Lowry base the conjugate acid and the conjugate base c CO32 aq H2O l HCO3 aq Identify conjugate acid base pairs in an acid base reaction The Arrhenius definition of acid and base is limited to aqueous that is water solutions
H3O is the conjugate acid of H2O since it can lose a proton in the reverse reaction The stronger an acid the weaker its conjugate base will be and the