Calculating Average Atomic Mass Worksheet Pdf Answers Sep 20 2022 0183 32 The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element Calculations of atomic mass use the percent abundance of each isotope
Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes each with 50 00 abundance One isotope has a mass of 63 00 amu and the other has a mass of 68 00 amu b Recalculate the atomic mass if instead there is 80 00 of the 63 00 amu isotope and 20 00 of the 68 00 amu isotope The relative abundance and atomic masses are 69 for mass of 62 30 for mass of 64 Calculate the average atomic mass of Copper Show ALL work for full credit Calculate the average atomic mass of Sulfur if 95 of all Sulfur atoms have a mass of 31 u 0 has a mass of 32 and 4 have a mass of 33 Show ALL work for full credit
Calculating Average Atomic Mass Worksheet Pdf Answers
 Calculating Average Atomic Mass Worksheet Pdf Answers
Calculating Average Atomic Mass Worksheet Pdf Answers
https://ecdn.teacherspayteachers.com/thumbitem/Calculating-Average-Atomic-Mass-2165018-1657562510/original-2165018-4.jpg
Calculate the average atomic mass of Sulfur if 95 00 of all sulfur atoms have a mass of 31 972 amu 0 76 have a mass of 32 971amu and 4 22 have a mass of 33 967amu The four isotopes of Lead are shown below each with its percent by mass abundance and the composition of its nucleus
Pre-crafted templates use a time-saving service for developing a varied variety of documents and files. These pre-designed formats and designs can be utilized for various individual and expert projects, including resumes, invitations, flyers, newsletters, reports, presentations, and more, streamlining the material creation procedure.
Calculating Average Atomic Mass Worksheet Pdf Answers

Calculating Average Atomic Mass Worksheet - Name ___Lisa Calculating - Studocu

Practice - Average Atomic Mass Worksheet 1.1 - Answer Key by The Chem Teacher
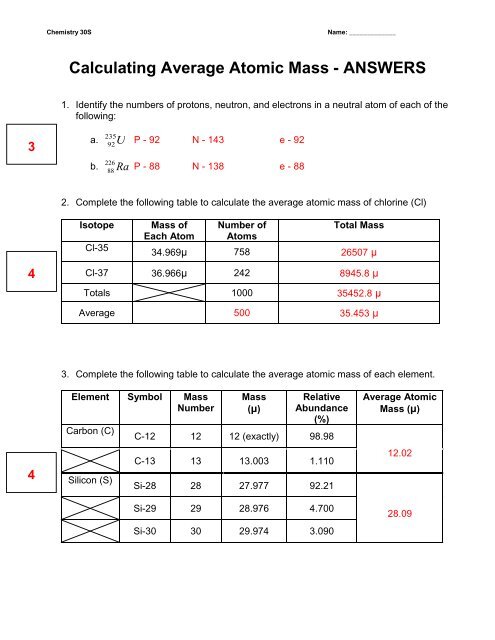
Calculating Average Atomic Mass - WC Miller Collegiate

Calculating Average Atomic mass Worksheet-1 1 .docx - Calculating Average Atomic Mass 2C Worksheet: 1. Three isotopes of Silicon occur in | Course Hero

Calculating Average Atomic Mass Chemistry Homework Worksheet | TPT

Kami Export - Mallak Aheel - Average atomic mass worksheet.pdf - Calculating Average Atomic Mass Worksheet: 1 Three isotopes of Silicon occur in | Course Hero

https://www.npsd.k12.nj.us/cms/lib04/NJ01001216
The average atomic mass of the three isotopes is 24 3050 amu If the atomic mass of 25Mg is 24 98584 amu and 26Mg is 25 98259 amu calculate the actual atomic mass of 24Mg Atomic Mass Total 9 22 Average Atomic Mass Worksheet Solutions 1 Rubidium has two common isotopes 85Rb and 87Rb

https://www.dentonisd.org//avg_atomic_mass_ws.pdf
To calculate average atomic mass of an element Average atomic mass fractional abundance of isotope 1 atomic mass of isotope 1 fractional abundance of isotope 2 atomic mass of isotope 2 Practice Problems 1 Chlorine has two isotopes Chlorine 35 has an actual mass of 34 9689 u and chlorine 37 has a mass of 36 9659 u

https://chem.libretexts.org/Courses/Oregon
An element has the following natural abundances and isotopic masses 90 92 abundance with 19 99 amu 0 26 abundance with 20 99 amu and 8 82 abundance with 21 99 amu Calculate the average atomic mass of this element Answer Click here to
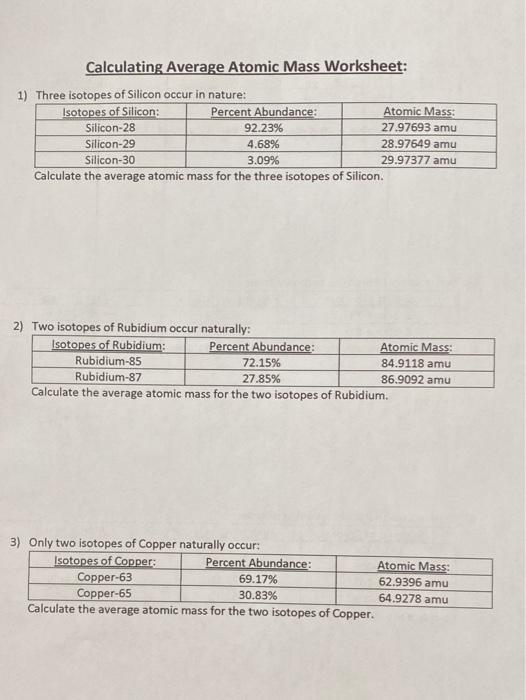
https://www.studocu.com/en-us/document/the-city
Calculating Average Atomic Mass Worksheet Show ALL calculation setups The term average atomic mass is a weighted average and so is calculated differently from a normal average Explain how this type of average is calculated This average is calculated by summing masses of the element s isotopes

https://www.hasdk12.org/cms/lib3/PA01001366
a What is the average atomic mass of this element b Use your periodic table to determine which element this is 10 An element exists as 4 different isotopes 4 35 have a mass of 49 9461 amu 83 79 have a mass of 51 9405 amu 9 50 have a mass of 52 9407 amu and 2 36 have a mass of 53 9389 amu a What is the average atomic
The relative atomic mass Ar of atoms is the average mass of all the different isotopes of an element taking into account the amount of each isotope on a scale where 12C atoms have a mass of exactly 12 Imagine you have 90 balls with mass 200 g and 10 balls with mass 300 g The average mass of the balls is given by Download now Wome Answer Ke Pd ate Accelerated Chemistry Average Atomic Mass Worksheet Calculate the average atomic mass for each element based on the natural abundance of its isotopes 2 Find the average atomic mass for Li if 7 5 of Li atoms are Li with a mass of 6 015123 amu and 92 5 are Li with a mass of 7 0160041
Jan 12 2022 0183 32 Age 15 16 Level 10 Language English en ID 2231485 01 12 2022 Country code US Country United States School subject Chemistry 1061818 Main content Atomic Mass 2104157 Practice Calculating average atomic mass Other contents Average Atomic Mass Share Print Worksheet Finish