Calculating Percent Abundance Of Isotopes Worksheet isotope mass percent abundance For example suppose we want to find the percent abundance of chlorine isotopes 35Cl and 37Cl given
Atomic mass worksheet One has a mass of 10 0129 amu and a percent abundance of 19 91 percent and another isotope of 11 0093 amu with a percent abundance of Isotopes Percent Abundance and Average Atomic Mass For most elements Directions Calculate the weighted average atomic mass of each element from its
Calculating Percent Abundance Of Isotopes Worksheet
 Calculating Percent Abundance Of Isotopes Worksheet
Calculating Percent Abundance Of Isotopes Worksheet
https://study.com/cimages/videopreview/videopreview-full/4zsdrconvl.jpg
Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes each with 50 00 abundance One isotope has a mass of 63 00
Pre-crafted templates use a time-saving solution for producing a diverse series of files and files. These pre-designed formats and layouts can be utilized for various individual and expert tasks, consisting of resumes, invitations, flyers, newsletters, reports, discussions, and more, simplifying the content production procedure.
Calculating Percent Abundance Of Isotopes Worksheet
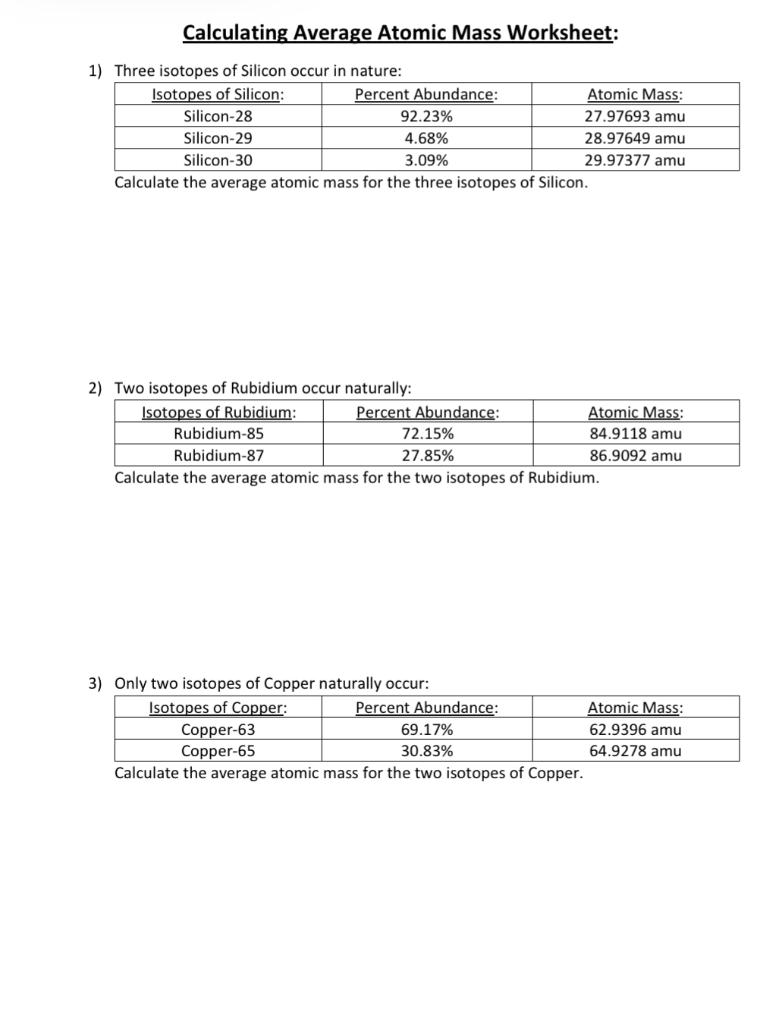
Solved Calculating Average Atomic Mass Worksheet: 1) Three | Chegg.com
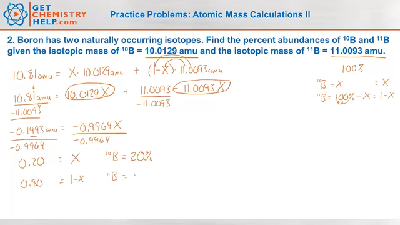
Chemistry Practice Problems: Atomic Mass Calculations II - Get Chemistry Help
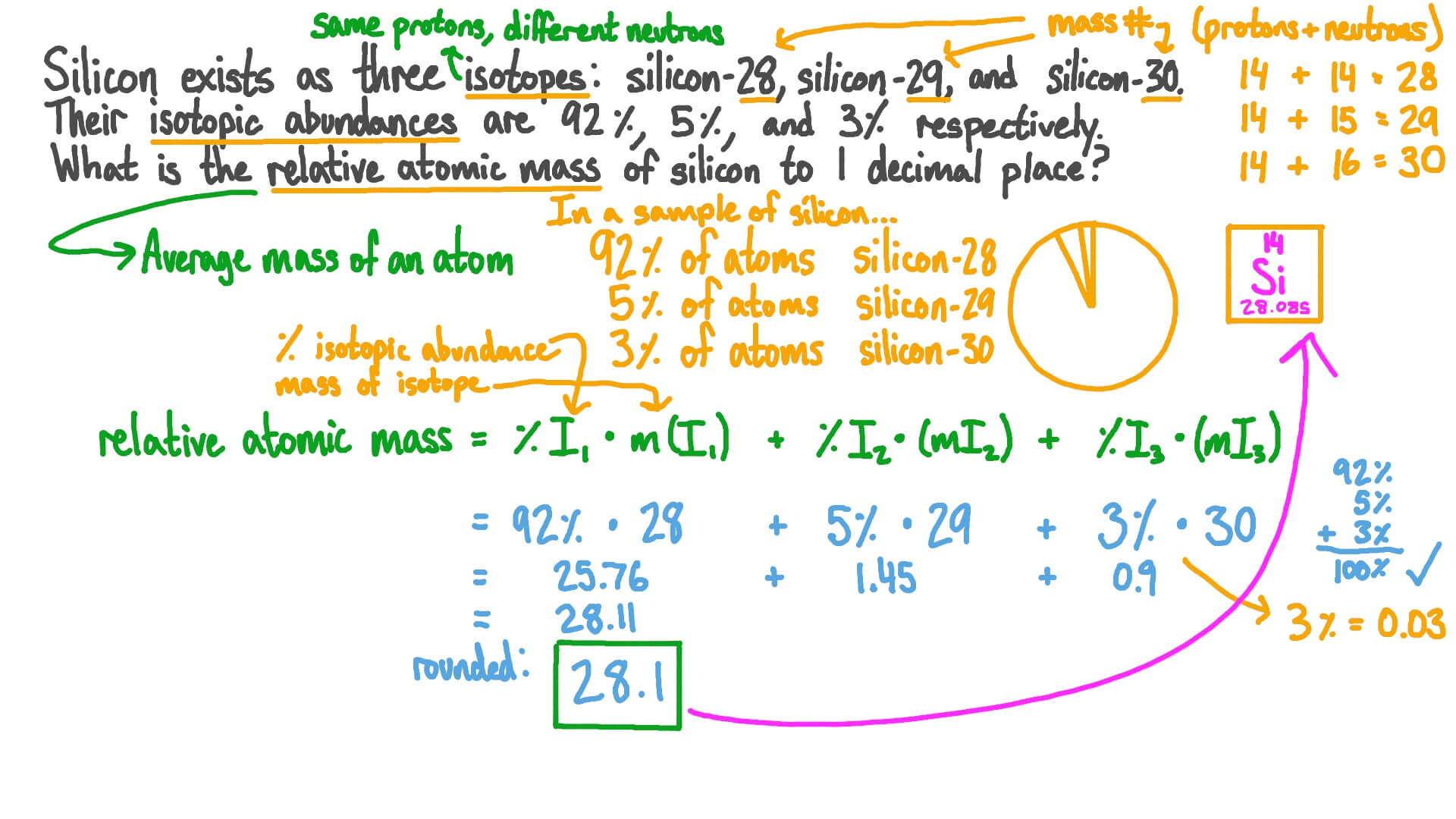
Question Video: Using Relative Abundance of Isotopes to Calculate Relative Atomic Mass | Nagwa

Isotopes Worksheet on Isotope Ratios Percent Abundance and Weighted Averages

Isotopes - relative abundance calculations structured worksheet | Teaching Resources

Atomic Mass Calculations

http://www.vaxasoftware.com/doc_eduen/qui/x_aver_atom_mass_isot.pdf
WORKSHEET GENERATORS www vaxasoftware pc Problems of If the average atomic mass of lutetium is 174 967 u calculate its percent isotopic abundances

https://www.livingston.org/cms/lib9/NJ01000562/Centricity/Domain/688/averageatomicmasshomeworkkey.doc
Determine the relative abundance of each isotope if the average atomic mass of copper is 63 55amu 63Cu 69 00 65Cu 31 00

https://www.chemteam.info/Mole/AvgAtomicWt-Reverse.html
Calculate the isotopic abundances when given the average atomic weight and the isotopic weights 1 Calculate the percent abundance Bl 55 77 0 7143 Br 15

https://issr.edu.kh/science/AS%20and%20IB%20Worksheets/Spectroscopy/Mass%20Spectrometry/!1%20Starter%20AS%20-%20Isotopic%20abundance.pdf
The data below gives the m z ratio and relative abundance of different isotopes of an element X Determine the relative atomic mass of the element X to 1 d p

https://www.nagwa.com/en/worksheets/793191041943/
Explore and practice Nagwa s free online educational courses and lessons for math and physics across different grades available in English for Egypt
Want to ace chemistry Access the best chemistry resource at http www conquerchemistry Get confident with calculating the relative abundance of isotopes from mass spectrometry data
1 The element copper has naturally occurring isotopes with mass numbers of 63 and 65 The relative abundance and atomic masses are 69 2 and 30 8 respectively