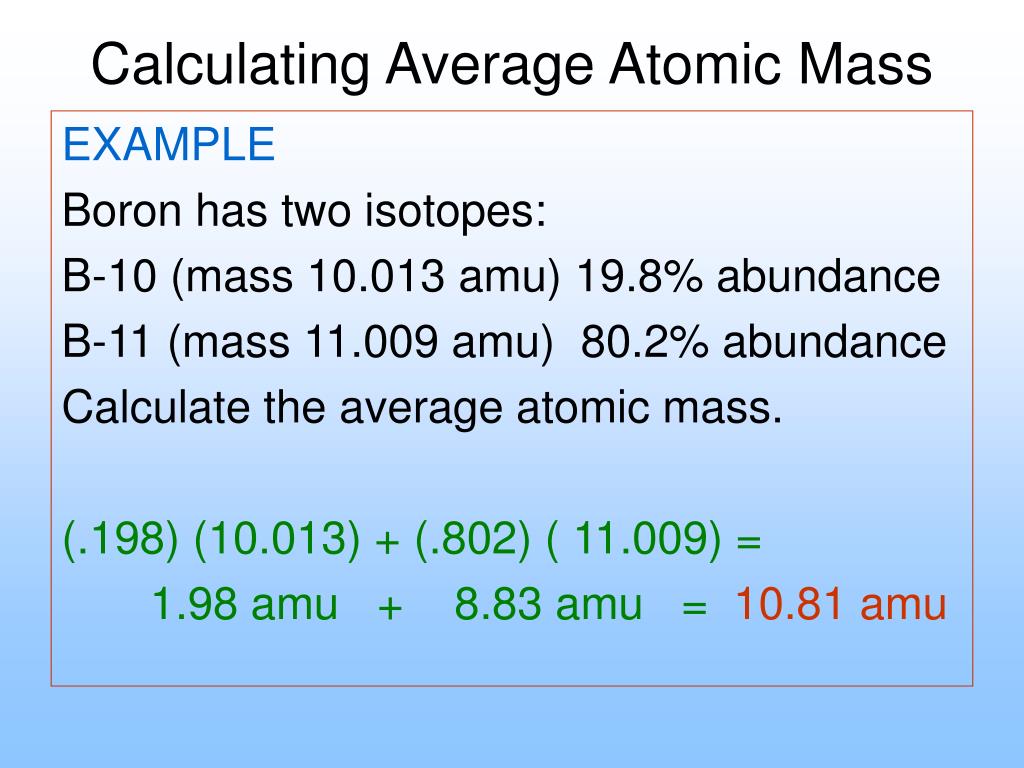
Chapter 5 Calculating Average Atomic Mass Worksheet Answers Sep 20 2022 0183 32 Were you to simply calculate the arithmetic average of the precise atomic masses you would get 36 frac left 34 969 36 966 right 2 35 968 text amu nonumber Clearly the actual average atomic mass from the last column of the table is significantly lower Why
The term average atomic mass is a average and so is calculated differently from a normal average Explain how this type of average is calculated The element Copper has naturally occurring isotopes with mass numbers of 63 and 65 The relative abundance and atomic masses are 69 for mass of 62 30 for mass of 64 Chapter 5 Worksheet 2 Isotopes and Average Atomic Mass Elements come in a variety of isotopes meaning they are made up of atoms with the sa me atomic number number of protons but different atomic mass numbers These atoms differ i n the number of neutrons The average atomic mass is the weighted average of all the isotopes of an element
Chapter 5 Calculating Average Atomic Mass Worksheet Answers

https://imgv2-1-f.scribdassets.com/img/document/431483964/original/daa43c3ebc/1631366183?v=1
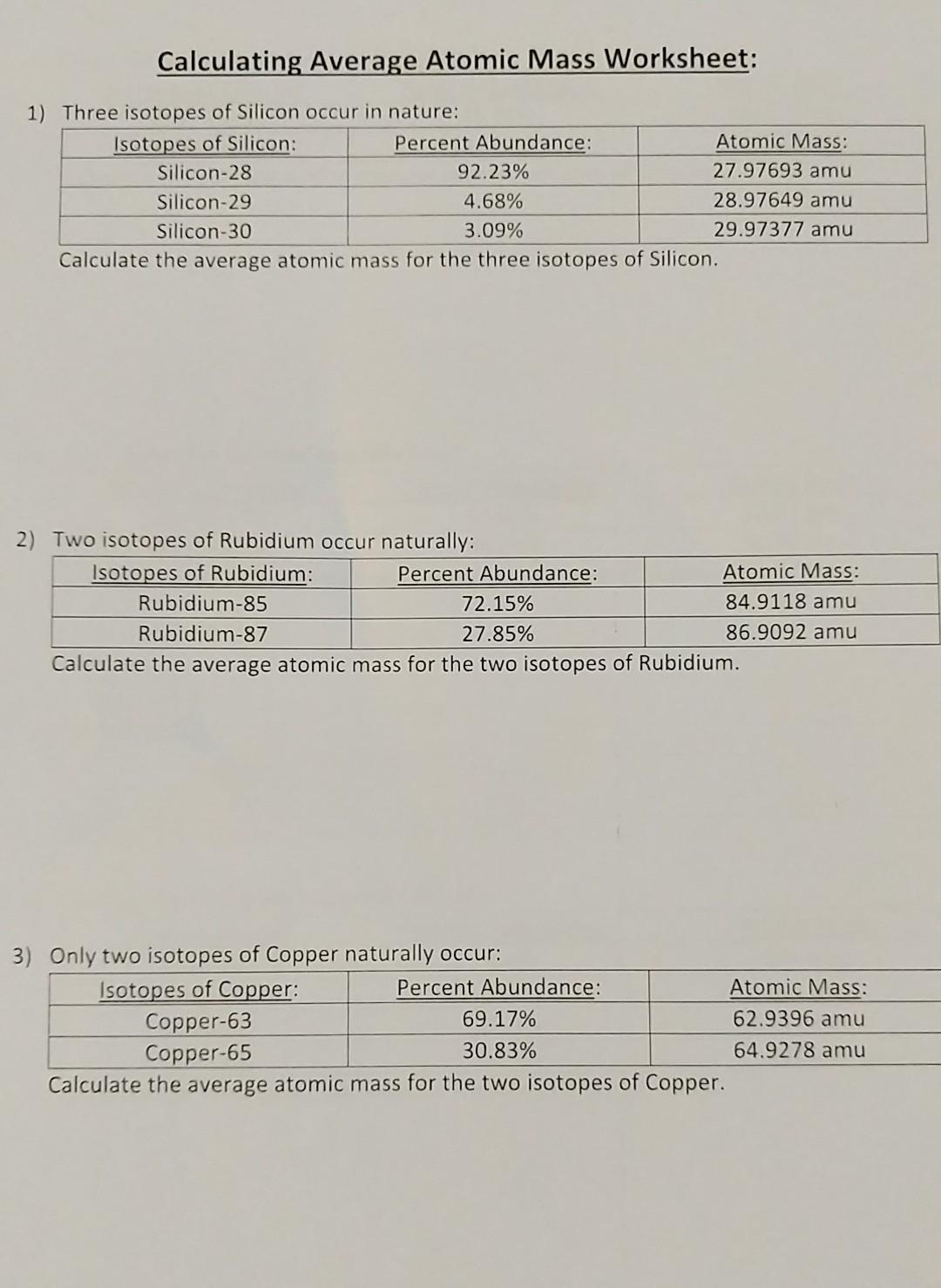
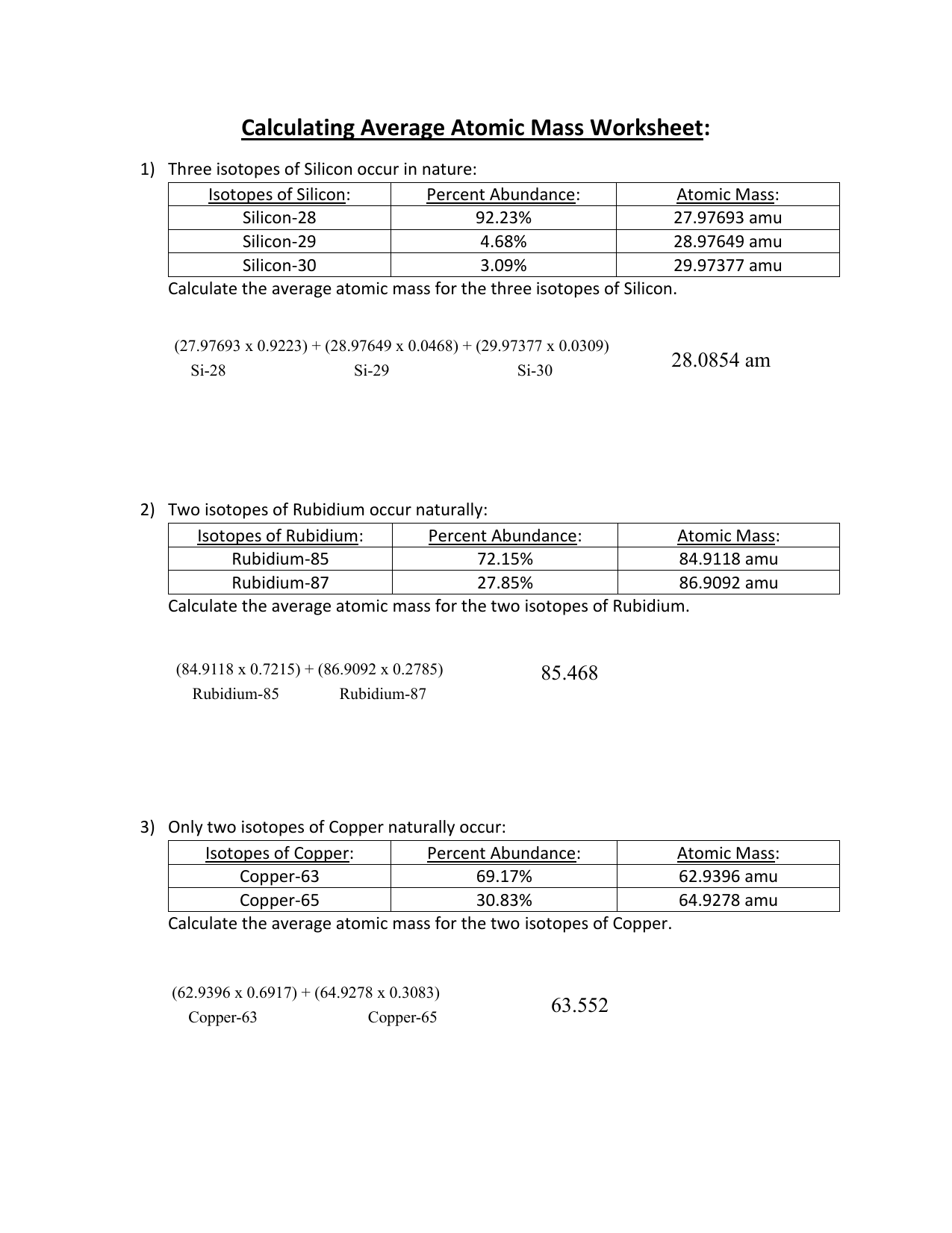
Calculating Average Atomic Mass Worksheet 1 Three isotopes of Silicon occur in nature Atomic Mass 27 97693 amu 28 97649 amu 29 97377 amu Isoto es of Silicon Silicon 28 Silicon 29 Silicon 30 Percent Abundance 92 23 4 68 3 09 Calculate the average atomic mass for the three isotopes of Silicon 9 Y o zq q 7377 0 0
Pre-crafted templates provide a time-saving solution for developing a diverse range of files and files. These pre-designed formats and designs can be utilized for different personal and expert tasks, including resumes, invites, flyers, newsletters, reports, discussions, and more, simplifying the content development process.
Chapter 5 Calculating Average Atomic Mass Worksheet Answers
PPT Average Atomic Mass PowerPoint Presentation Free Download ID

40 Calculating Atomic Mass Worksheet Answers Worksheet Master
Average Atomic Mass Worksheet Answers

Understanding The Average Atomic Mass Worksheet Free Worksheets

Atomic Structure Review Worksheet Atomic Size Worksheet Printable Vrogue
Information Average Atomic Mass

https://quizlet.com/522669915/chemistry-average
Calculate the average atomic mass for each element based on the natural abundance of its isotopes Learn with flashcards games and more for free

https://www.studocu.com/en-us/document/the-city
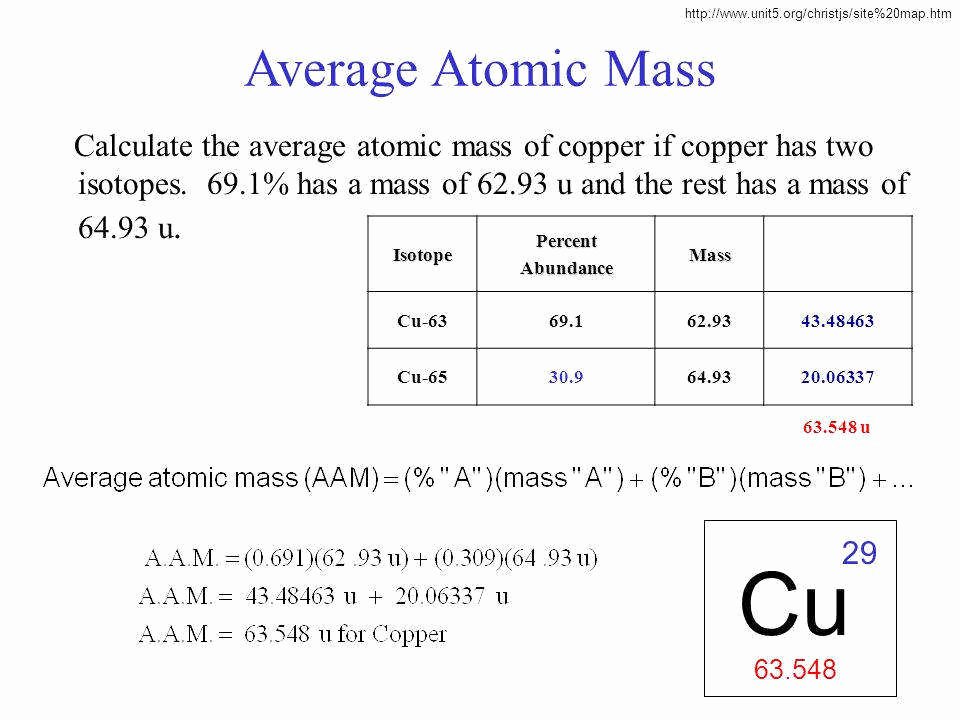
The element Copper has naturally occurring isotopes with mass numbers of 63 and 65 The relative abundance and atomic masses are 69 for mass of 62 30 for mass of 64 Calculate the average atomic mass of Copper Show ALL work for full credit 69 100 x 62 43 30 100 x 64 19 ggghvhvhvhvhhv 63

https://www.scribd.com/document/431483964/Average
Average Atomic Mass Worksheet Answer Key Free download as PDF File pdf or read online for free
https://www.dentonisd.org//avg_atomic_mass_ws.pdf
Practice Problems 1 Chlorine has two isotopes Chlorine 35 has an actual mass of 34 9689 u and chlorine 37 has a mass of 36 9659 u In any sample of chlorine atoms 75 771 will be chlorine 35 and 24 229 will be chlorine 37 Calculate the average atomic mass of chlorine 2 Copper has two isotopes
https://www.hasdk12.org/cms/lib3/PA01001366
Calculate the average atomic mass of the element Iodine I using the following data Isotope abundance Iodine 127 80 Iodine 126 17 Iodine 128 3 8 Calculate the average atomic mass of the element Hydrogen H using the following data Isotope abundance Hydrogen 1 99 Hydrogen 2
Answer 152 04 amu 4 Strontium consists of four isotopes with masses of 84 abundance 0 50 86 abundance of 9 9 87 abundance of 7 0 and 88 abundance of 82 6 Calculate the atomic mass of strontium Answer 87 71 amu 5 Titanium has five common isotopes 46Ti 8 0 47Ti 7 8 48Ti 73 4 49Ti 5 5 50Ti 5 3 Calculating Average Atomic Mass High School Chemistry Skills Practice 1 Carbon has three isotopes namely Carbon 12 Carbon 13 and Carbon 14 C 12 has a mass of 12 000 amu and is 98 89
Calculate the average atomic mass 6 Copper used in electric wires comes in two flavors isotopes 63Cu and 65Cu 63Cu has an atomic mass of 62 9298 amu and an abundance of 69 09 The other isotope 65Cu has an abundance of 30 91 The average atomic mass between these two isotopes is 63 546 amu Calculate the actual atomic mass of