Chemistry Unit 4 Worksheet 4 More Empirical Formulas Answers NAME CLASS INTRODUCTION The empirical formula of a compound is defined as the simplest whole number ratio of atoms of the elements in the compound The molecular formula of a compound is defined as the actual number of atoms of elements covalently bonded in a molecule and is a whole number ratio of the empirical formula
Jul 21 2022 0183 32 Determining Empirical Formulas An empirical formula tells us the relative ratios of different atoms in a compound The ratios hold true on the molar level as well Thus H 2 O is composed of two atoms of hydrogen and 1 atom of oxygen Likewise 1 0 mole of H 2 O is composed of 2 0 moles of hydrogen and 1 0 mole of oxygen May 5 2017 0183 32 File previews docx 16 26 KB docx 2 74 MB Students will learn simple techniques on how to solve problems involving empirical formula and molecular formula from this worksheet Tes paid licence How can I reuse this Report this resource to let us know if it violates our terms and conditions Our customer service team will review your
Chemistry Unit 4 Worksheet 4 More Empirical Formulas Answers
 Chemistry Unit 4 Worksheet 4 More Empirical Formulas Answers
Chemistry Unit 4 Worksheet 4 More Empirical Formulas Answers
https://askworksheet.com/wp-content/uploads/2021/04/eba3d7451df51dffbb3eb18cac1869d9.jpg
1 4 1 4 Empirical formula NH 4 SO 4 Step 2 use MR to find molecular formula MR mass NH 4 SO 4 x n 228 114 x n n 2 moloecular formula N 2 H 8 S 2 O 8 QUESTION 1 A substance contains 40 carbon 6 hydrogen and 53 oxygen its MR is 60 Find its molecular formula Step 1 calculate empirical formula ATOMS C H O
Templates are pre-designed documents or files that can be used for various purposes. They can conserve time and effort by providing a ready-made format and layout for creating various sort of material. Templates can be used for individual or professional jobs, such as resumes, invitations, leaflets, newsletters, reports, discussions, and more.
Chemistry Unit 4 Worksheet 4 More Empirical Formulas Answers
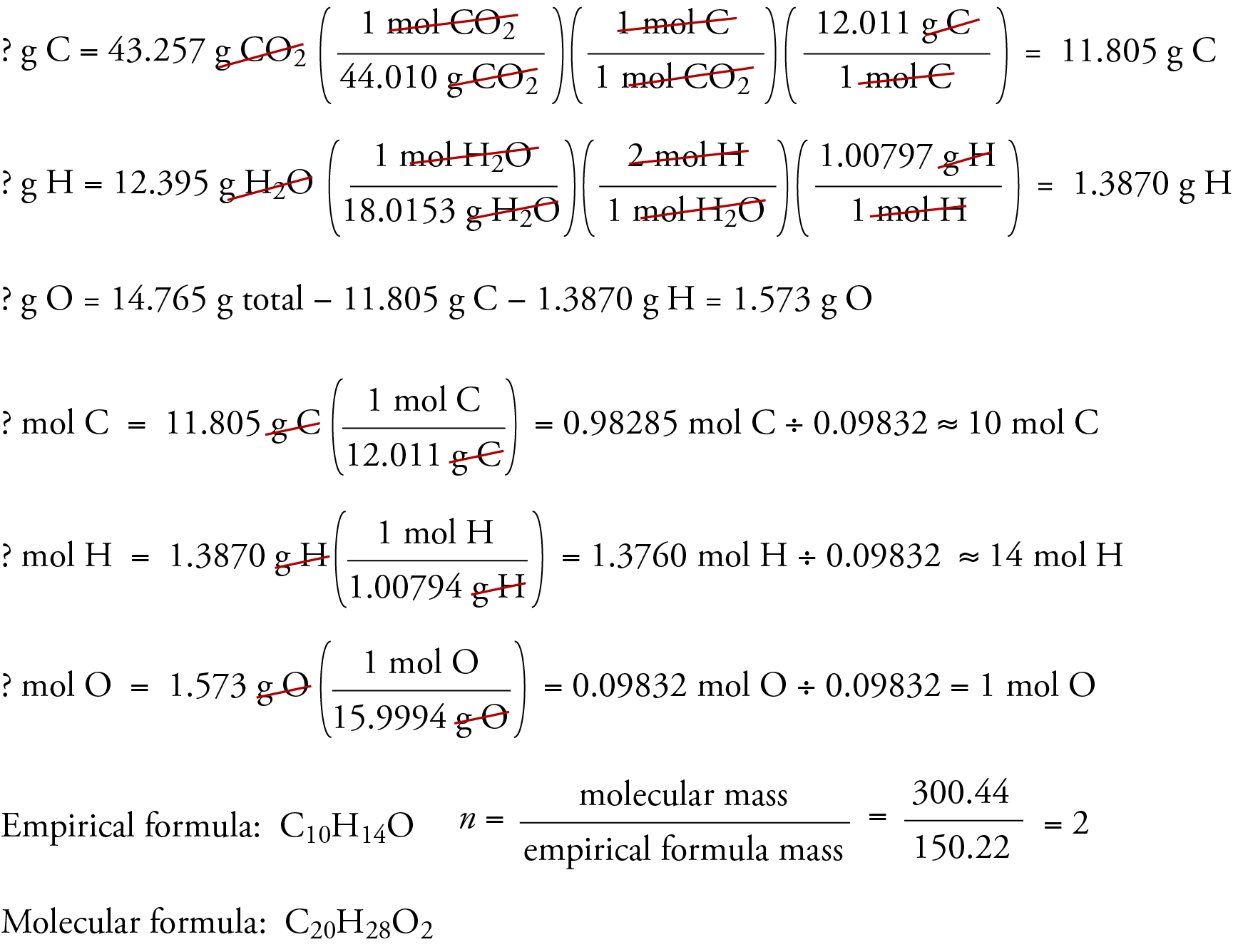
Combustion Analysis

Unit 2 Worksheet 1 Chemistry Answers Db excel

Chemistry UNIT 4 Notes PDF

A2 Level Chemistry Unit 5 Revision Notes En Apple Books

Empirical And Molecular Formula Worksheet

Chemistry Unit 4 Worksheet 3 Language Worksheet Pictures 2020

https://chem.libretexts.org/Courses/Oregon
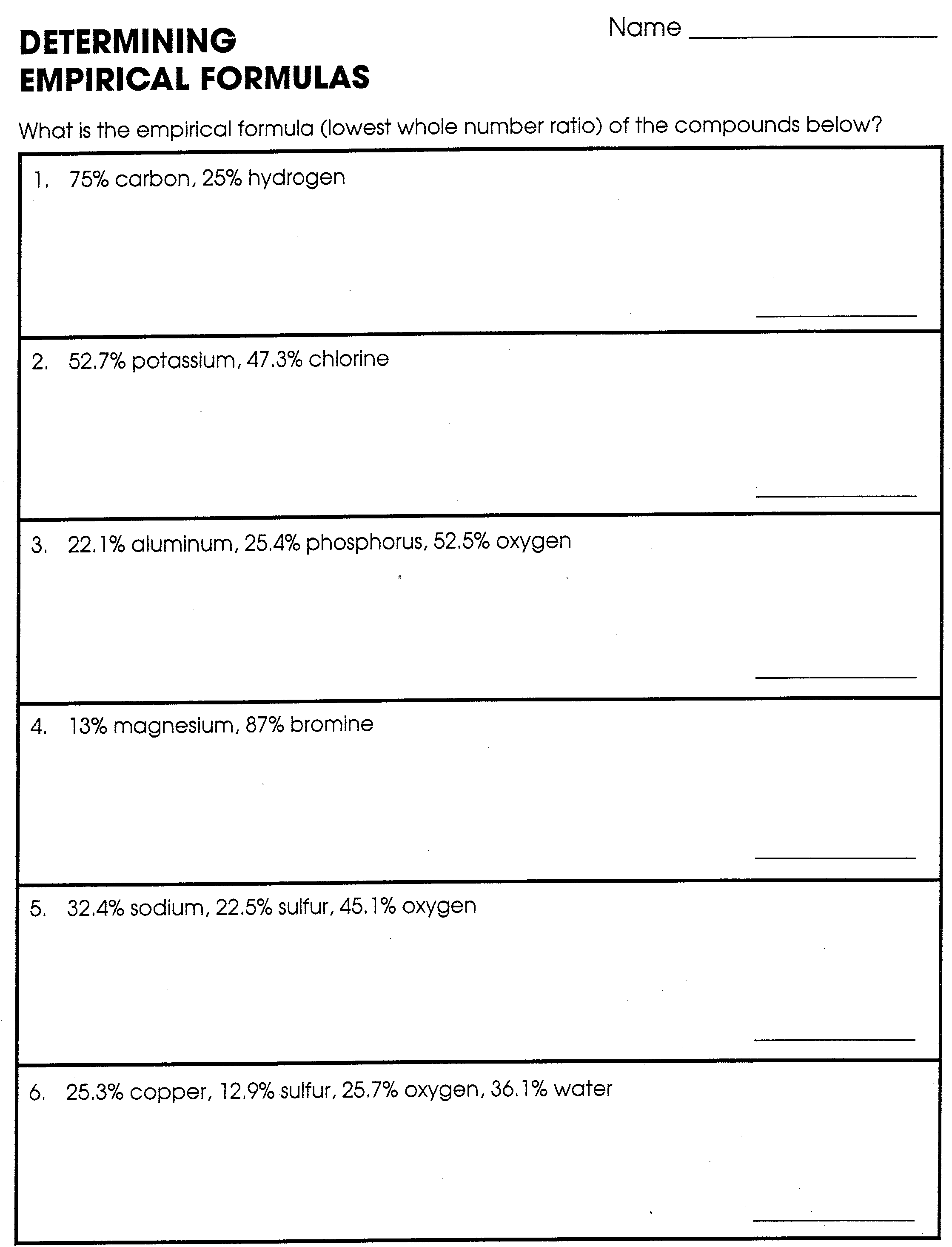
Determine the empirical formulas for compounds with the following percent compositions a 15 8 carbon and 84 2 sulfur b 40 0 carbon 6 7 hydrogen and 53 3 oxygen Answer a Answer b Click here to see a video of the solution

https://chem.libretexts.org/Courses/Oregon_Tech
May 28 2020 0183 32 Determine the empirical formulas for compounds with the following percent compositions a 15 8 carbon and 84 2 sulfur b 40 0 carbon 6 7 hydrogen and 53 3 oxygen Answer a Answer b Click here to see a video of the solution
https://openstax.org/books/chemistry-2e/pages/3-2
As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula If the molecular or molar mass of the substance is known it may be divided by the empirical formula mass to yield the number of empirical formula units per molecule n

https://cpb-ap-se2.wpmucdn.com/learn.stleonards
Empirical and molecular formula calculations Let the unknown metal be X As it is in group 1 the empirical formula must be XCl There is 47 6 Cl so the percentage composition of X must be 52 4 X 39 02 and the formula is KCl Pearson Education Australia a division of Pearson Australia Group Pty Ltd 2008
https://www.thoughtco.com/empirical-formula
Jul 3 2019 0183 32 This 10 question practice test deals with finding empirical formulas of chemical compounds A periodic table will be required to complete this practice test Answers for the test appear after the final question Question 1 What is the empirical formula of a compound containing 40 0 sulfur and 60 0 oxygen by mass Question 2
Empirical formula worksheet To print or download this file click the link below Empirical Formula Worksheet pdf PDF document 1 83 MB 1921394 bytes As the name suggests an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula If we know the molecular or molar mass of the substance we can divide this by the empirical formula mass in order to identify the number of empirical formula units per molecule which we designate as n
Learn about the AP Chemistry essentials These are things that you should have memorized to succeed in AP Chemistry We ll cover everything from nomenclature to energy to significant figures With this review you ll be more than ready to