Determining Empirical Formulas Worksheet Empirical Formula 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical
Determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g mol 0 8435 1 08435 10 139C 12 01g mol They are tested on various problems ranging from a simple calculation of percent composition to analyzing practical situations Students are expected to
Determining Empirical Formulas Worksheet
 Determining Empirical Formulas Worksheet
Determining Empirical Formulas Worksheet
https://www.coursehero.com/thumb/2d/fe/2dfefda240216bab915ba63980d2dff2a76dbc33_180.jpg
Worksheet Composition and Name Empirical Formulas CHEMISTRY A Study of Matter 2004 GPB 7 21 Part 1 Determine the empirical formula
Pre-crafted templates use a time-saving option for developing a varied series of documents and files. These pre-designed formats and designs can be used for different personal and expert jobs, consisting of resumes, invitations, leaflets, newsletters, reports, presentations, and more, improving the material development process.
Determining Empirical Formulas Worksheet

Free Printable Percent Composition and Empirical Formula Worksheets

Using Percent Composition to Determine Empirical Formulas worksheet

Empirical Formula Worksheet II | PDF
Mole Assign & Key #2 -

Calculating Empirical Formulas Worksheet by All Your Chemistry Needs

Chapter 8 Empirical and Molecular Formulas Worksheet 1 Key PDF | PDF

https://web1.tvusd.k12.ca.us/gohs/myoung/Chem.%2017-18/Emp%20and%20Mol%20Formula%20Calcs%20answers.pdf
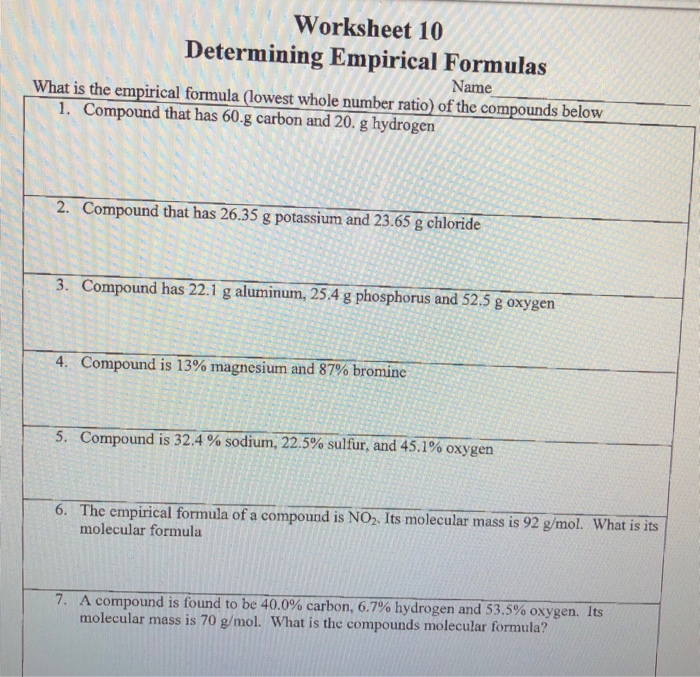
DETERMINING MOLECULAR FORMULAS TRUE FORMULAS Solve the problems below Name KEY 1 The empirical formula of a compound is NO Its molecular mass is 92 g
https://justonly.com/chemistry/chem201/students/worksheets/empirical_molecular.pdf
Empirical Molecular Formula Practice Worksheet Directions Find Determine the empirical and molecular formula of a compound composed of 18 24 g Carbon

https://mrskerrscience.weebly.com/uploads/3/7/2/1/37215807/empirical_formula_worksheet-answers.pdf
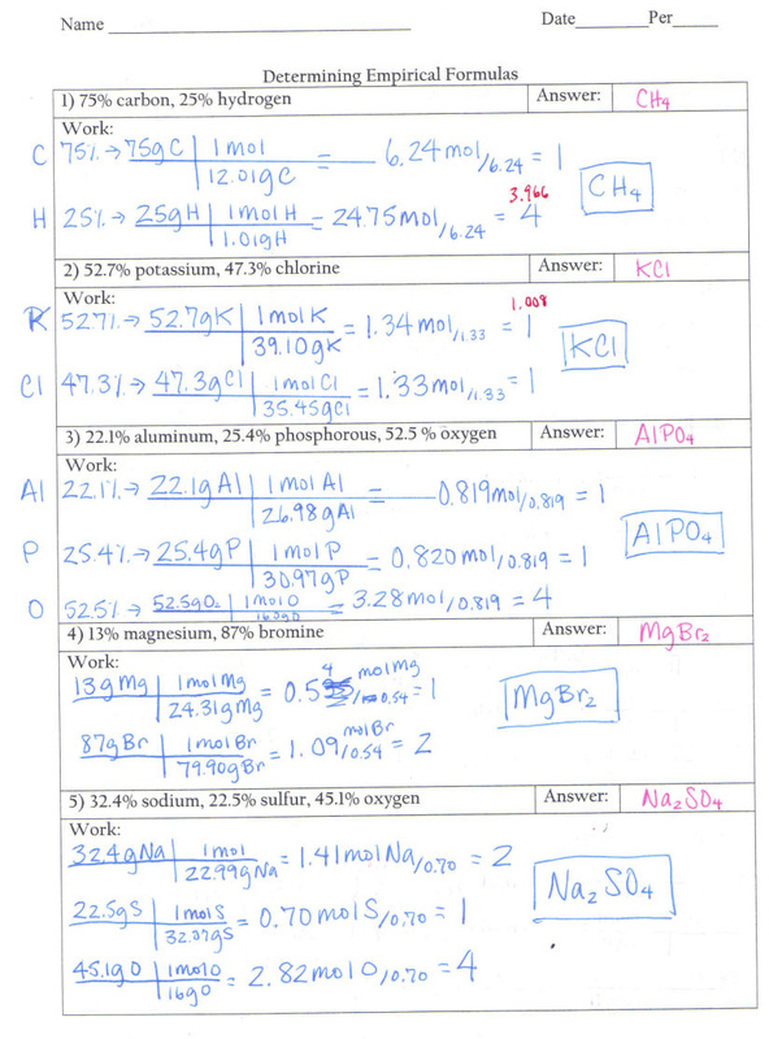
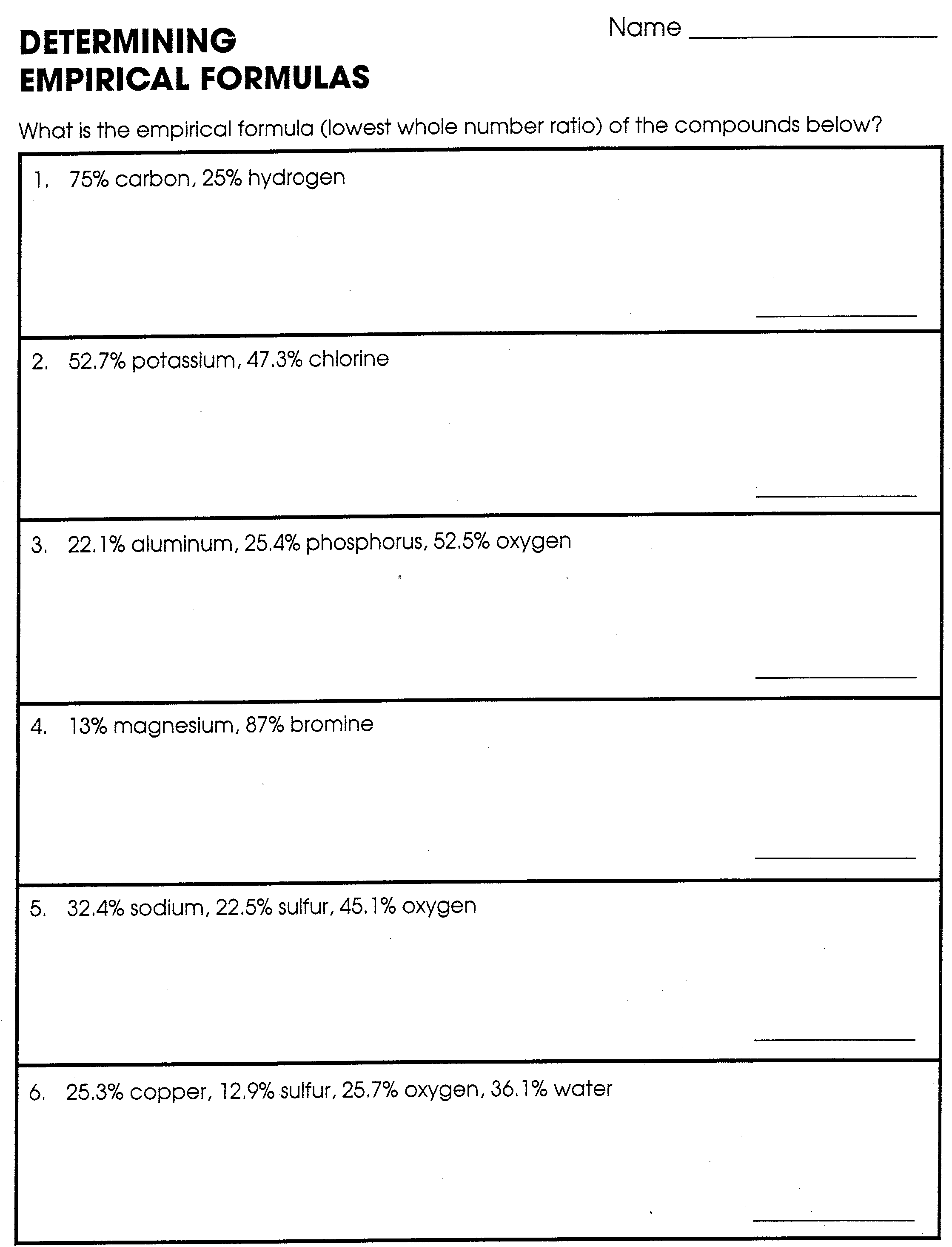
Determining Empirical Formula What is the empirical formula lowest whole number ratio of the compounds below 1 75 carbon 25 hydrogen 2 52 7
https://learningcentre.vcc.ca/media/vcc-library/content-assets/learning-centre/worksheets/by-coursex2fprogram/mathx2fscience/Chem0861-EmpiricalFormulasAndMolecularFormulas.pdf
EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if
https://www.wylieisd.net/cms/lib09/TX01918453/Centricity/Domain/2398/Empirical%20Formula%20Practice.pdf
Empirical Formula a formula showing the smallest whole number mole ratio 1 Convert the grams of each element to moles 2 To find the simplest ratio divide
You can determine the empirical formula for any compound as long as you know the mass of each element present the percentage of mass for each present element Thank you very much for downloading Determining Empirical Formulas Worksheet As you may know people have look hundreds times for their chosen readings
Deriving the number of moles of each element from its mass Dividing each element s molar amount by the smallest molar amount to yield