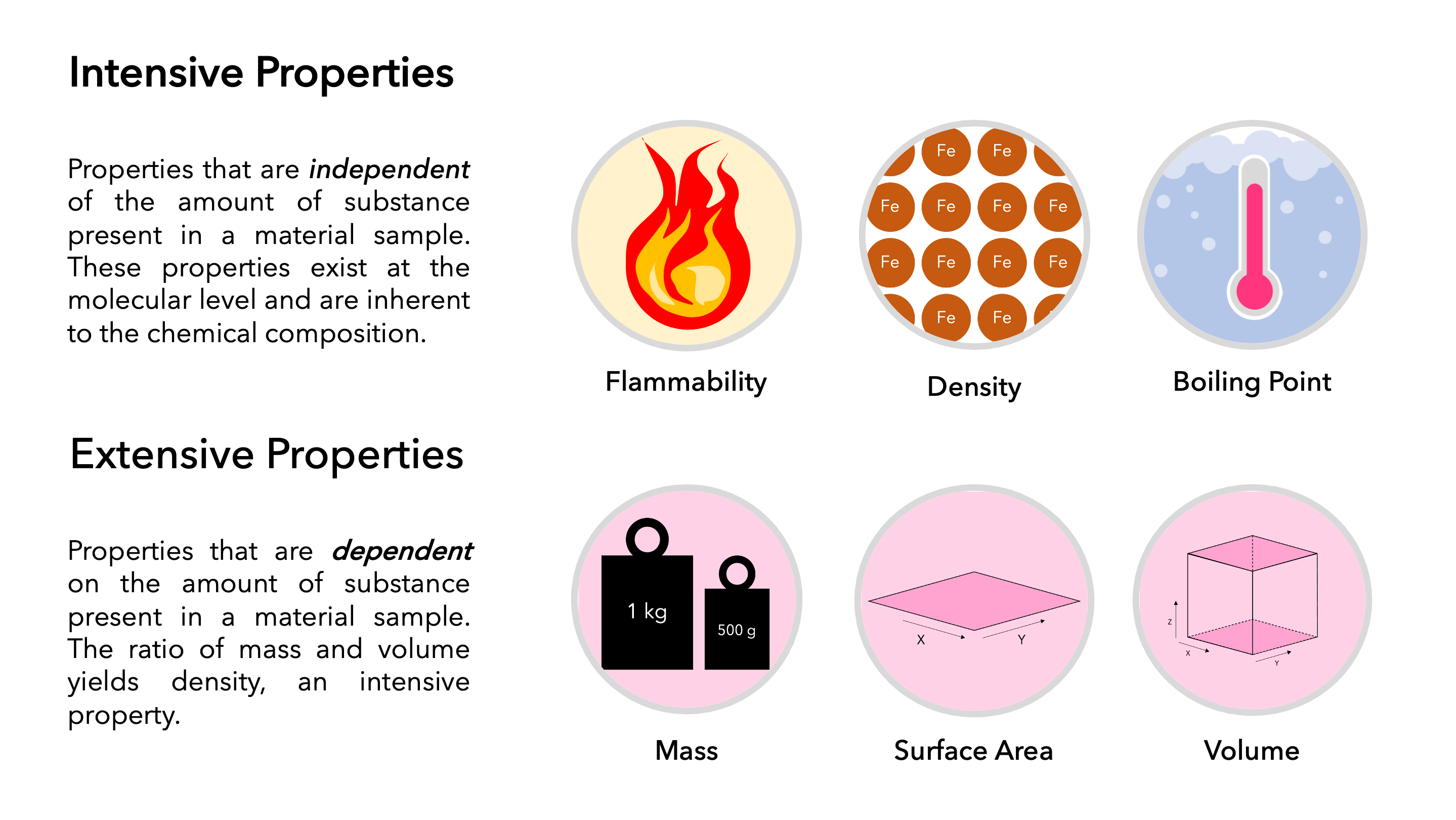
Difference Between Intensive And Extensive Properties With Example Jan 5 2024 0183 32 Frequently Asked Questions The Main Difference between Intensive and Extensive Intensive vs Extensive Key Takeaways Intensive properties do not depend on the amount of matter and include characteristics like density and color Extensive properties are dependent on the sample size and include mass volume and length
Examples Color Hardness Density Freezing boiling point The freezing point of 1 kg water is 273 K What is the freezing point of 2 kg of water Solution Since freezing point is intensive it does not change as the amount of a substance changes Therefore the freezing point of any quantity of water is 273 K Extensive properties Jan 29 2024 0183 32 1 Basic Concepts and Definitions 1 4 Extensive and intensive properties
Difference Between Intensive And Extensive Properties With Example
 Difference Between Intensive And Extensive Properties With Example
Difference Between Intensive And Extensive Properties With Example
https://d20khd7ddkh5ls.cloudfront.net/intensive_vs_extensive.png
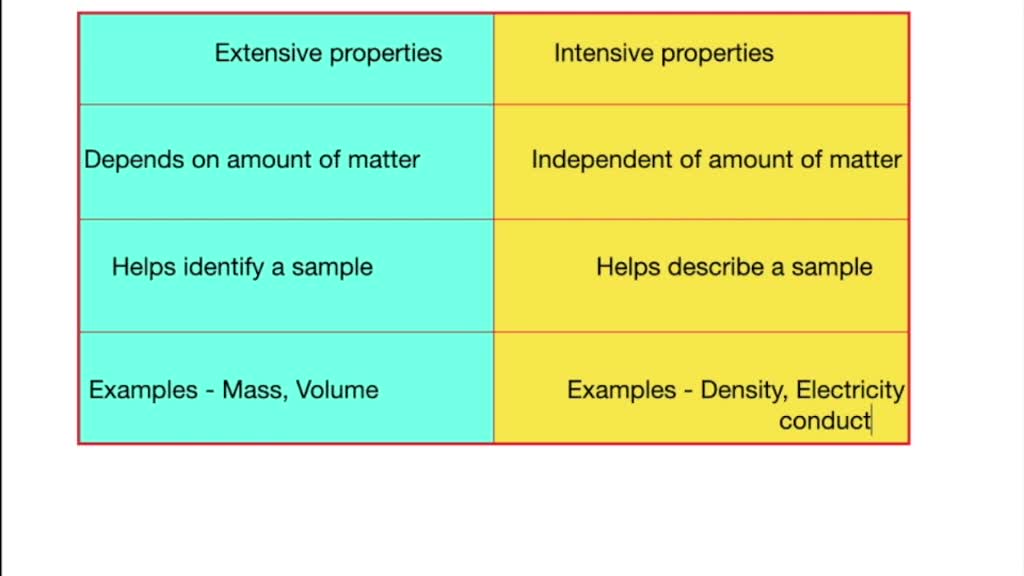
Jul 31 2023 0183 32 Intensive Vs Extensive Properties INTENSIVE EXTENSIVE Doesn t depend on size Depends on size Remains constant Varies with size Cannot be calculated Can be measured Easy to identify Not easily identifiable Examples Temperature density colour Examples Volume mass length
Templates are pre-designed documents or files that can be used for various purposes. They can conserve time and effort by offering a ready-made format and layout for creating different kinds of material. Templates can be utilized for individual or expert projects, such as resumes, invites, leaflets, newsletters, reports, discussions, and more.
Difference Between Intensive And Extensive Properties With Example
SOLVED What Is The Difference Between Extensive Properties And

Is The Weight Of A System An Extensive Or Intensive Property

Extensive And Intensive Properties YouTube

Extensive Vs Intensive Properties Difference And Comparison

Chapter 1 Section 2 Intensive And Extensive Properties New YouTube

Difference Between Intensive And Extensive Properties FDB

https://chem.libretexts.org/Bookshelves
Summary An extensive property is a property that depends on the amount of matter in a sample Mass and volume are examples of extensive properties An intensive property is a property of matter that depends only on the type of matter in

https://www.thoughtco.com/intensive-vs-extensive
Dec 4 2019 0183 32 Key Takeaways Intensive vs Extensive Properties The two types of physical properties of matter are intensive properties and extensive properties Intensive properties do not depend on the quantity of matter Examples include density state of matter and temperature Extensive properties do depend on sample size

https://sciencenotes.org/intensive-extensive-properties
Mar 18 2020 0183 32 Extensive and intensive properties are the two types of physical properties of matter Intensive properties do not depend on the amount of matter in a substance Examples include state of matter temperature and density Extensive properties depend on the amount of matter in a sample Examples include mass length

https://en.wikipedia.org/wiki/Intensive_and_extensive_properties
Examples of intensive properties include temperature T refractive index n density and hardness By contrast an extensive property or extensive quantity is one whose magnitude is additive for subsystems Examples include mass volume and entropy Not all properties of matter fall into these two categories
/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png?w=186)
https://chem.libretexts.org/Courses/can/CHEM_210
Dec 13 2023 0183 32 Physical properties can be extensive or intensive Extensive properties vary with the amount of the substance and include mass weight and volume Intensive properties in contrast do not depend on the amount of the substance they include color melting point boiling point electrical conductivity and physical state at a given temperature
Dec 15 2017 0183 32 1 What are Intensive Properties Definition Examples 2 What are Extensive Properties Definition Examples 3 What is the Difference Between Intensive and Extensive Properties Comparison of Key Differences Key Terms Boiling Point Bulk Properties Density Energy Extensive Intensive Matter Melting Point Physical Intensive properties are independent of the amount of substance present Or they are bulk properties Characteristic doesn t change The size of intensive properties does not change Density temperature pressure etc are some examples of intensive properties Extensive Properties
Jan 15 2024 0183 32 Intensive property is a property of matter that does not change with the size of the sample For example pressure density etc Extensive property is a property of matter that depends upon the amount of substance i e varies with the size of the material like weight volume mass etc