Electronegativity And Bond Polarity Worksheet Answers Activity 09 2 The positively charged protons in the nucleus attract the negatively charged electrons As the number of protons in the nucleus increases the electronegativity or attraction will increase
Solved Example for You Q How does the electronegativity vary along the Group 14 elements Ans As we move down the group the electronegativity decreases in general The reason Correct option is D F gt O gt N gt C Electronegativity increases across a period with increase in atomic number Hence fluorine is most electronegative and carbon is least electronegative
Electronegativity And Bond Polarity Worksheet Answers Activity 09 2
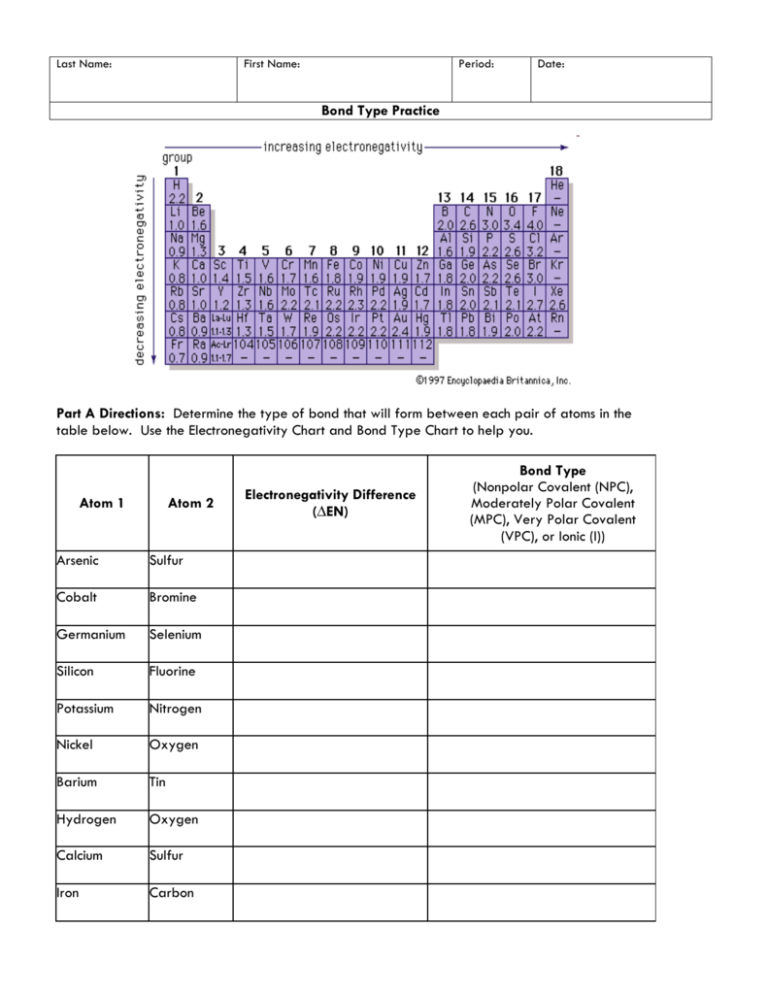 Electronegativity And Bond Polarity Worksheet Answers Activity 09 2
Electronegativity And Bond Polarity Worksheet Answers Activity 09 2
https://s3.studylib.net/store/data/007680294_2-b6a5db141cb22b753af8dbaf59ddbb46-768x994.png
In a covalent bond the relative ability of each atom to attract the bonded pair of electrons towards itself is called electronegativity
Templates are pre-designed documents or files that can be utilized for various purposes. They can conserve time and effort by offering a ready-made format and design for producing different kinds of material. Templates can be used for individual or professional projects, such as resumes, invitations, leaflets, newsletters, reports, discussions, and more.
Electronegativity And Bond Polarity Worksheet Answers Activity 09 2

Covalent Bonding Practice Worksheets

I Need Help Pls Activity 2 Determining Polarity Of Molecules After

Worksheets Polarity Of Bonds

IMFs And Polarity Worksheet

Electronegativity And Bond Polarity A Chemistry Worksheet Made By

Exam Gizmos Student Exploration Polarity And Intermolecular Forces

https://www.toppr.com › ask › question
Which of the following is the correct decreasing order of atomic radius for following elements B Al Ga In

https://www.toppr.com › ask › question › what-is-electronegativity-how-d…
The ability of an atom to attract the shared electron pair of a covalent bond in a molecule towards itself is called electronegativity In a period from left to right the electronegativity

https://www.toppr.com › ask › question
In group 13 electronegativity first decreases from B to Al and then increase marginally down the group This is because of non metallic nature of B discrepancies in atomic size of element

https://www.toppr.com › ask › question › in-cno-and-f-theelectronigativity
Correct option is B Increases from carbon to fluorine Electronegativity increases across a period with increase in atomic number Hence fluorine is most electronegative and carbon is least

https://www.toppr.com › ask › question › the-order-of-electronegativity-fo…
The correct option is A F gt CI gt Br gt I It decreases on moving down the group because size of atom increases Decreasing order of electro negativity of halogens is F Cl Br I Fluorine has
[desc-11] [desc-12]
[desc-13]