Electronegativity Chart Worksheet Answers Electronegativity increases across a period with increase in atomic number Hence fluorine is most
Correct option is C Electronegativity increases as the s character in hybrid orbital increases Effective nuclear charge is directly proportional to electronegativity The positively charged Define the term Electronegativity State its unit View Solution
Electronegativity Chart Worksheet Answers
 Electronegativity Chart Worksheet Answers
Electronegativity Chart Worksheet Answers
https://static.zerochan.net/Jyahnar.full.3942636.png
The order of electronegativity of carbon in s p s p 2 and s p 3 hybridized states follows View Solution Q5
Pre-crafted templates use a time-saving solution for producing a varied variety of files and files. These pre-designed formats and layouts can be made use of for various personal and expert projects, consisting of resumes, invites, leaflets, newsletters, reports, discussions, and more, streamlining the material development procedure.
Electronegativity Chart Worksheet Answers
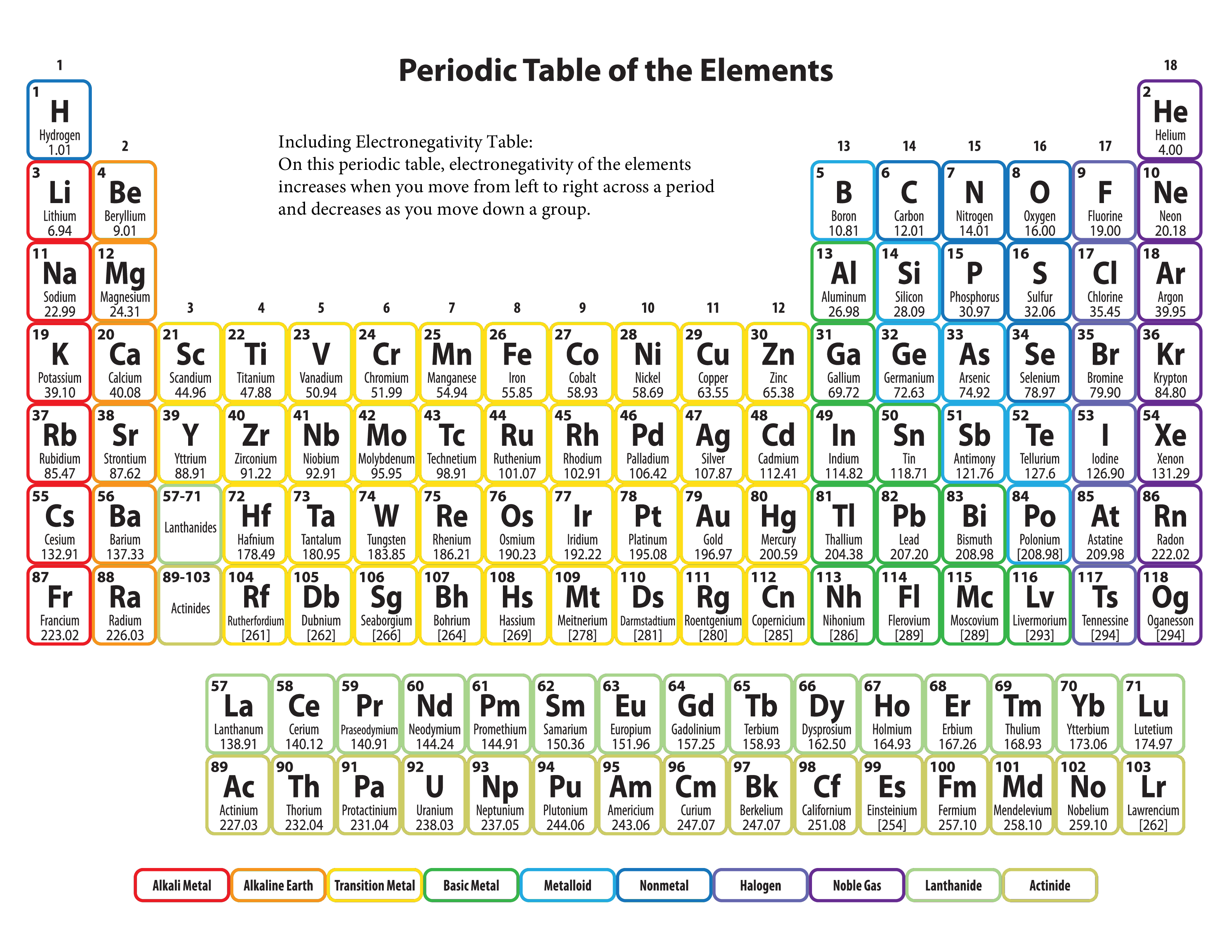
Electronegativity Chart Printable

30 Interesting Riddles For Adults Challenge Your Brain Now Funny

Kiana Kaslana Houkai 3rd Image By HoYoverse 3942637 Zerochan

Houraiji Kyuushou Kyuushou Houraiji Houkai Gakuen Image By

Electronegativity Chart 3 Free Templates In PDF Word Excel Download
.PNG)
Periodic Table Of Electronegativities

https://www.toppr.com › ask › question
Click here to get an answer to your question electronegativity of group 13 elements follow the order

https://www.toppr.com › ask › question › what-is-electronegativity-how
The ability of an atom to attract the shared electron pair of a covalent bond in a molecule towards itself is called electronegativity In a period from left to right the electronegativity

https://www.toppr.com › ask › question › in-cno-and-f-theelectronigativity
Electronegativity increases across a period with increase in atomic number Hence fluorine is most electronegative and carbon is least electronegative Was this answer helpful

https://www.toppr.com › ask › question › the-order-of-electronegativity
The correct option is A F gt CI gt Br gt I It decreases on moving down the group because size of atom increases Decreasing order of electro negativity of halogens is F Cl Br I Fluorine has

https://www.toppr.com › guides › chemistry › the-p-block-elements › gro…
Q How does the electronegativity vary along the Group 14 elements Ans As we move down the group the electronegativity decreases in general The reason behind this irregularity is
[desc-11] [desc-12]
[desc-13]