Electronegativity Worksheet Key Give the trends of atomic radius ionic radius ionisation enthalpy electron gain enthalpy electronegativity of s p d f block elements View Solution View Solution View Solution
Electronegativity is a measure of the ability of an atom in a molecule to draw bonding electrons to itself Electronegativity generally increases from left to right on the periodic table and What is basic difference between the terms electron gain enthalpy electronegativity and Ionization enthalpy Give example of each View Solution Q 3
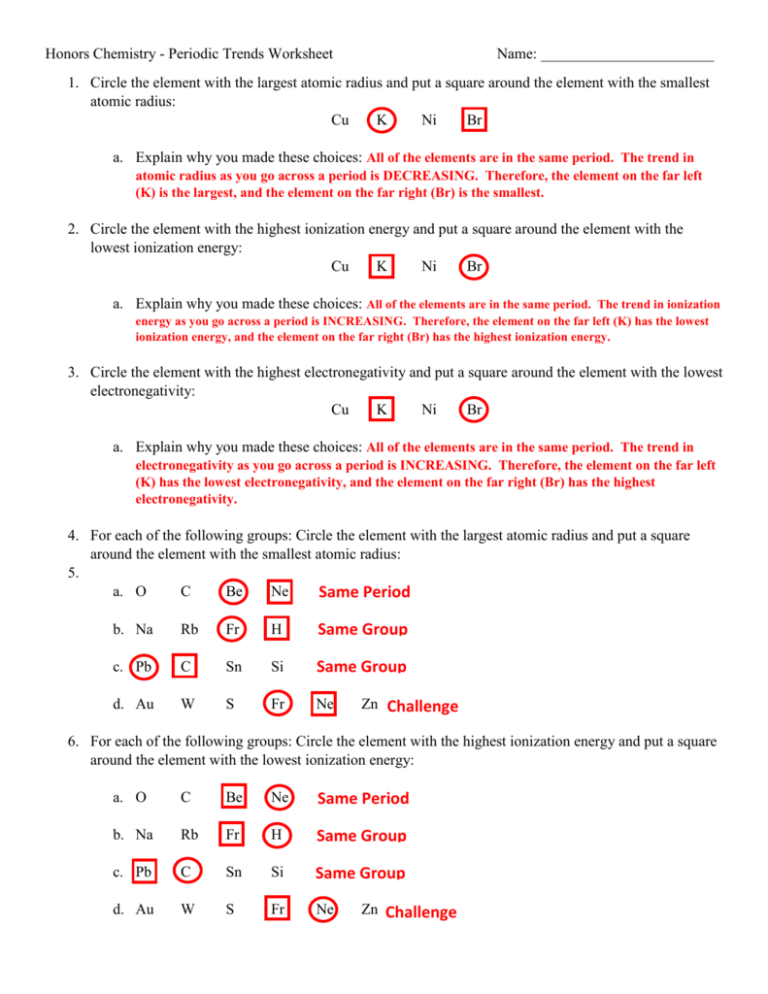
Electronegativity Worksheet Key
 Electronegativity Worksheet Key
Electronegativity Worksheet Key
https://worksheets.clipart-library.com/images2/electronegativity-worksheet/electronegativity-worksheet-11.png
Electronegativity is the tendency of an atom to attract the shared pair of electrons towards it in a covalent bond It is the property of atoms in molecules Electron gain enthalpy is the tendency
Pre-crafted templates provide a time-saving solution for developing a diverse series of files and files. These pre-designed formats and designs can be made use of for various personal and expert projects, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, simplifying the material production process.
Electronegativity Worksheet Key

Ratio Worksheet Maths Academy

Periodic Table Electronegativity Trend Ionization Energy

Polarity And Electronegativity Worksheets
Periodic Table Trends Worksheet Answers Printable Word Searches

Periodic Trends Atomic Radius Worksheet Printable Word Searches

How To Find Polarity Using Electronegativity

https://www.toppr.com › ask › question
Which of the following is the correct decreasing order of atomic radius for following elements B Al Ga In

https://www.toppr.com › ask › question › what-is-electronegativity-how-d…
The ability of an atom to attract the shared electron pair of a covalent bond in a molecule towards itself is called electronegativity In a period from left to right the electronegativity

https://www.toppr.com › ask › question
In group 13 electronegativity first decreases from B to Al and then increase marginally down the group This is because of non metallic nature of B discrepancies in atomic size of element

https://www.toppr.com › ask › question › in-which-of-the-following-the-or…
Electronegativity increases from left to right and decreases from top to bottom Thus in group 3 we have the order Si lt P lt S lt Cl and F gt Cl gt Br gt I Electronegativities of halogens are not in

https://www.toppr.com › ask › question
The order of electronegativity of carbon in sp sp2 and sp3 hybridized states follows
[desc-11] [desc-12]
[desc-13]