Empirical And Molecular Formula Practice Problems With Answers Pdf Empirical and Molecular Formulas Worksheet Objectives be able to calculate empirical and molecular formulas Empirical Formula 1 What is the empirical formula of a compound that
Empirical and Molecular Formula Practice Problems Determine the Empirical Formula for the following compounds Original Formula Empirical Formula 1 C 6 H 6 2 C 8 H 18 3 C 2 H 6 O 2 EMPIRICAL AND MOLECULAR FORMULAE PRACTISE QUESTIONS pdf Free download as PDF File pdf Text File txt or read online for free The document provides practice
Empirical And Molecular Formula Practice Problems With Answers Pdf
 Empirical And Molecular Formula Practice Problems With Answers Pdf
Empirical And Molecular Formula Practice Problems With Answers Pdf
https://s3.studylib.net/store/data/007884987_2-0d8a2dde770adff75de1113cf0fd4dd0.png
A compound has the empirical formula C3H6O and a molecular mass of 174 240 g mol What is the molecular formula of this compound Find the molar mass of the empirical formula
Templates are pre-designed files or files that can be used for various purposes. They can conserve time and effort by providing a ready-made format and layout for producing different sort of material. Templates can be used for individual or professional tasks, such as resumes, invitations, flyers, newsletters, reports, discussions, and more.
Empirical And Molecular Formula Practice Problems With Answers Pdf

Molecular Formula Practice Molecular Formula Worksheet Molecular
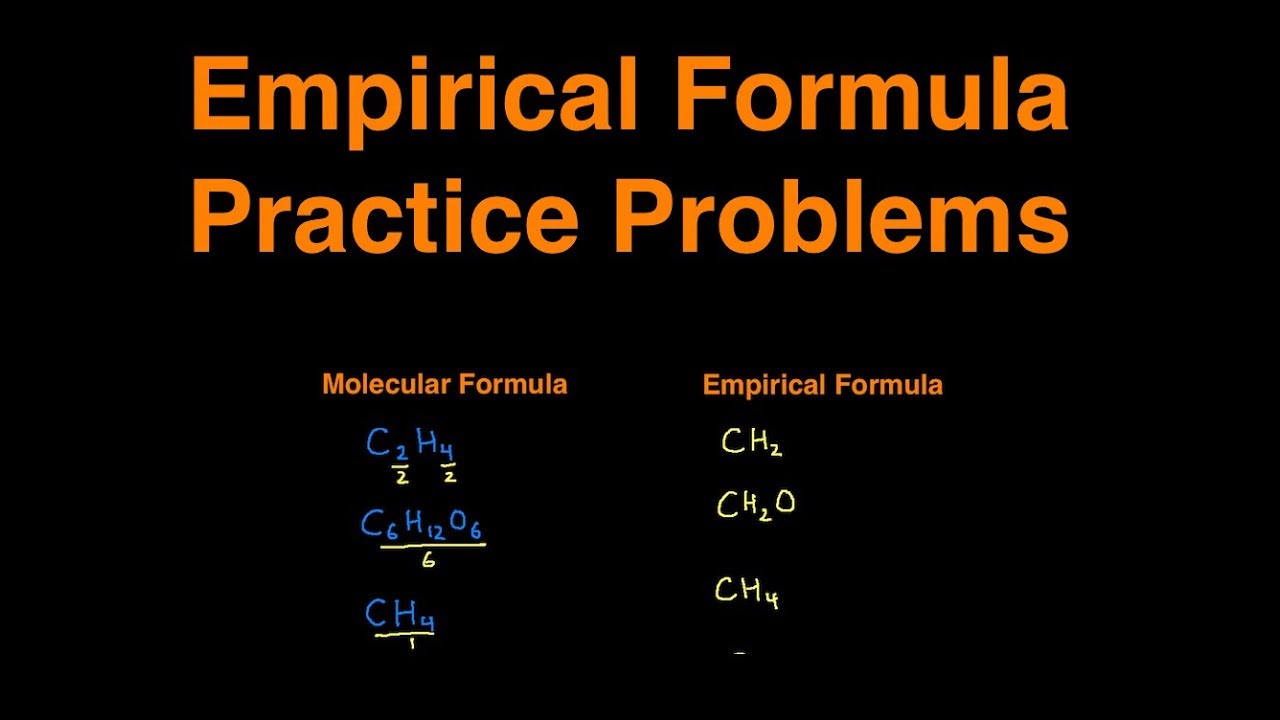
Empirical Formula Practice Problems Examples Practice Questions YouTube

Stoichiometry Worksheet 3 Gram To Gram Calculations Answers Askworksheet

Empirical And Molecular Formula Practice
Empirical Vs Molecular Formula

Empirical And Molecular Formulas Worksheet

https://chem.libretexts.org › Courses › Oregon_Tech
May 28 2020 0183 32 Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The

https://hschemsolutions.com › files › Download
Empirical and Molecular Formulas Worksheet 1 What is the empirical formula for C8H18 2 What is the empirical formula for H2O 3 What is the empirical formula for C4H10 4 What is

https://tsfx.edu.au › resources › W_-_Empirical
Write the empirical formula for the following compounds A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this
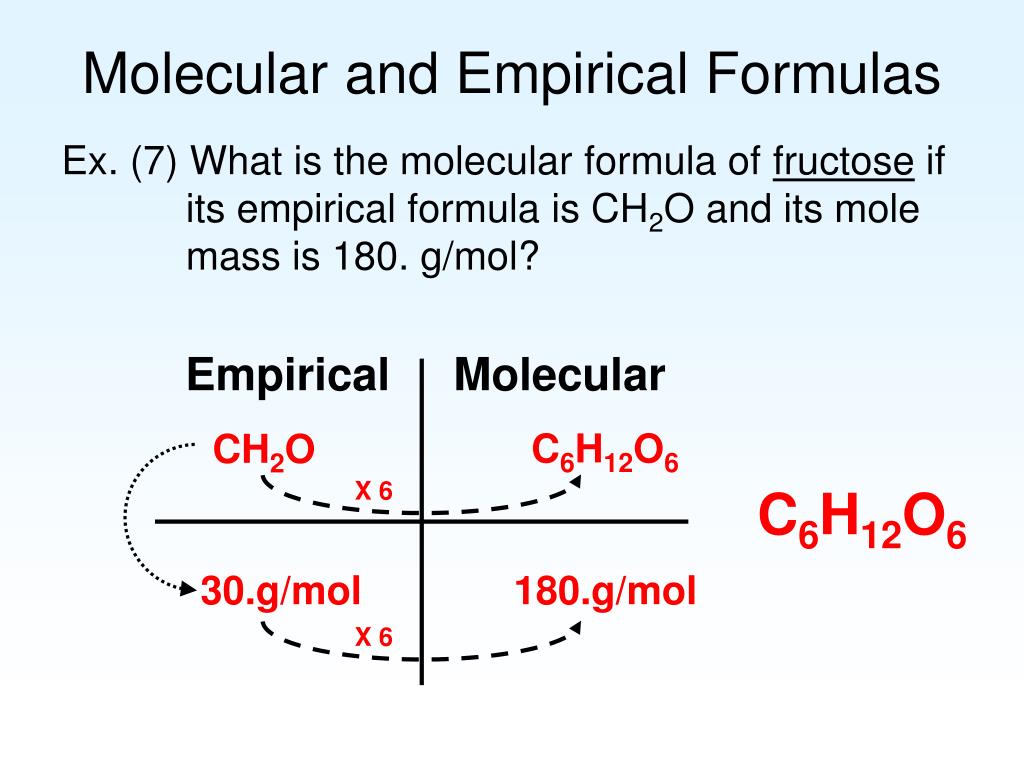
http://plaza.ufl.edu › ctoyota
Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in the problems are grams Step 1 convert to

https://www.justonly.com › › empirical_mo…
5 D e t e rm i ne t he e m pi ri c a l a nd m ol e c ul a r form ul a of a c om pound c om pos e d of 18 24 g C a rbon 0 51 g H ydroge n a nd 16 91 g F l uori ne ha s a m ol a r m a s s 562 0 g m
Empirical Formula Example Problems 1 A compound is analyzed and found to contain 79 8 g C and 20 2 g H Determine the empirical formula 2 What is the empirical formula of a compound Let the unknown metal be X As it is in group 1 the empirical formula must be XCl There is 47 6 Cl so the percentage composition of X must be 52 4 X 39 02 and the formula is KCl
EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if they have not already been