How To Do Bohr Atomic Models Sep 20 2022 0183 32 Bohr s Atomic Model Following the discoveries of hydrogen emission spectra and the photoelectric effect the Danish physicist Niels Bohr 1885 1962 proposed a new model of the atom in 1915 Bohr proposed that electrons do not radiate energy as they orbit the nucleus but exist in states of constant energy that he called stationary states
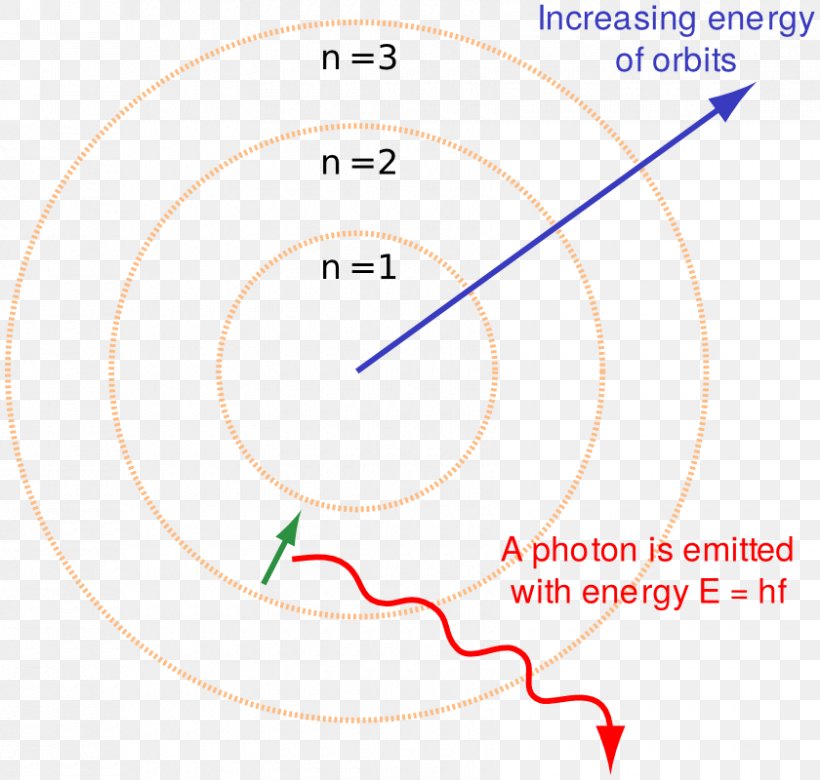
Jan 8 2024 0183 32 In the Bohr model of the atom electrons travel in defined circular orbits around the nucleus The orbits are labeled by an integer the quantum number n Electrons can jump from one orbit to another by emitting or absorbing energy The Bohr Model is a structural model of an atom The model was proposed by physicist Niels Bohr in 1913 In this model the electrons travel around the nucleus of an atom in distinct circular orbits or shells The model is also
How To Do Bohr Atomic Models
 How To Do Bohr Atomic Models
How To Do Bohr Atomic Models
https://sciencenotes.org/wp-content/uploads/2020/07/bohr-model2-1024x683.jpg
Jan 27 2020 0183 32 The Bohr Model has an atom consisting of a small positively charged nucleus orbited by negatively charged electrons Here s a closer look at the Bohr Model which is sometimes called the Rutherford Bohr Model Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915
Templates are pre-designed files or files that can be utilized for different purposes. They can conserve time and effort by providing a ready-made format and design for developing different kinds of material. Templates can be used for personal or professional tasks, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
How To Do Bohr Atomic Models

Refasmash blogg se Niels Bohr Atomic Model 3d
Bohr Atomic Model Of Hydrogen

Pin By Marla Stockert On Science Bohr Model Project Ideas Bohr Model

Interactive Atomic Model According To Bohr W19901 Atomic Models
Bohr Atomic Model 3d
Bohr Model Atomic Theory Rutherford Model Electron PNG 840x800px

https://www.khanacademy.org/science/physics/
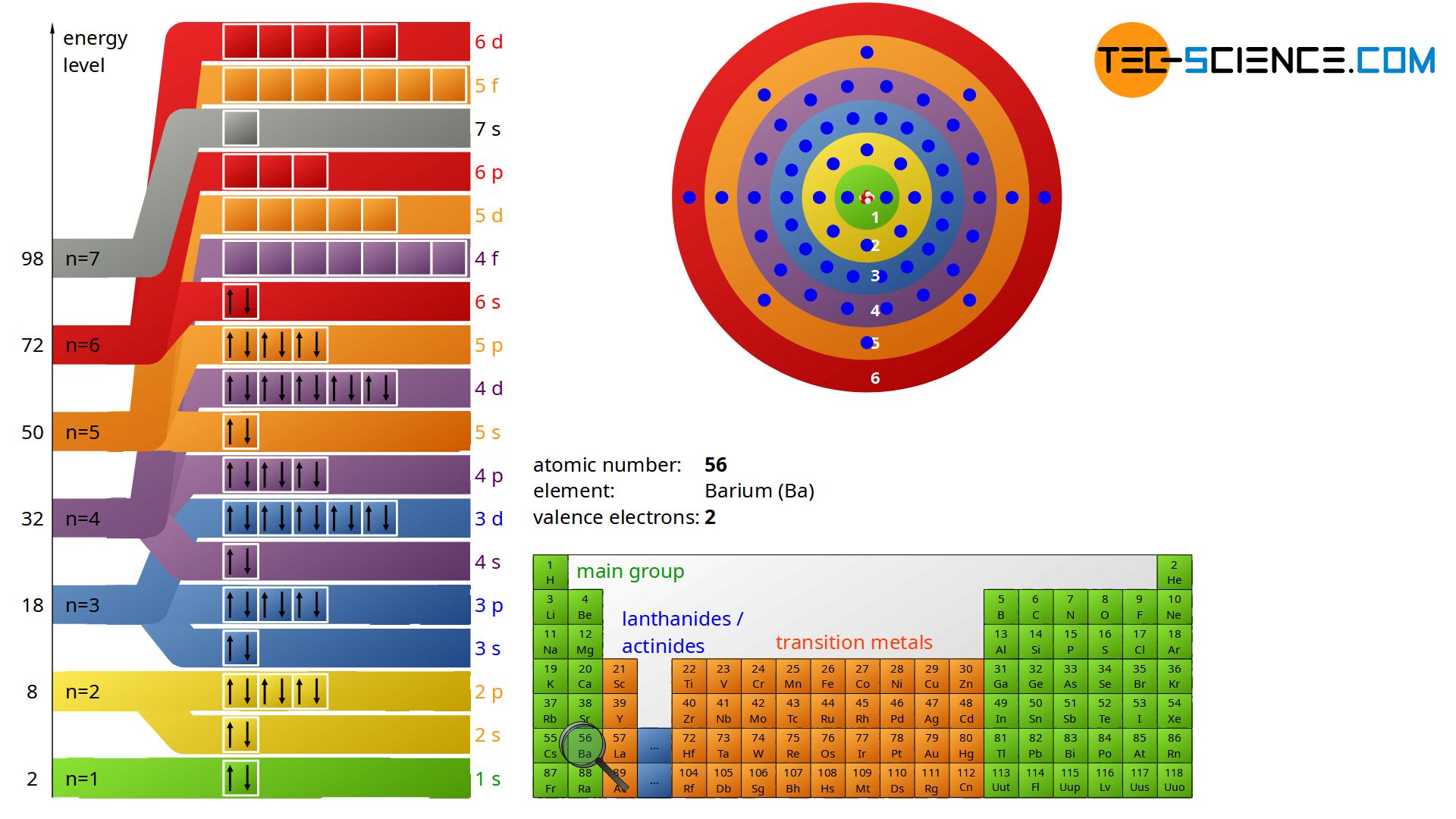
Bohr s model calculated the following energies for an electron in the shell n E n 1 n 2 13 6 eV Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels where the photon energy is h E 1 n l o w 2 1 n h i g h 2 13 6 eV

https://sciencing.com/make-bohr-model-atom-7729497.html
Apr 24 2017 0183 32 A Bohr model of an atom is a simplified visual representation of invisible atomic structures You can easily make a model of the complex and sometimes confusing interconnected relations of protons neutrons and electrons These models can help students visualize the fundamental principles of the electron orbits of quantum

https://chem.libretexts.org//Bohr_Diagrams_of_Atoms_and_Ions
Jan 30 2023 0183 32 Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun In the Bohr model electrons are pictured as traveling in circles at different shells depending on which element you have Figure PageIndex 2 contrast the Bohr diagrams for lithium fluorine and aluminum atoms

https://en.wikipedia.org/wiki/Bohr_model
In atomic physics the Bohr model or Rutherford Bohr model of the atom presented by Niels Bohr and Ernest Rutherford in 1913 consists of a small dense nucleus surrounded by orbiting electrons It is analogous to the structure of the Solar System but with attraction provided by electrostatic force rather than gravity and with the

https://blog.prepscholar.com/bohr-model
In order to make a Bohr diagram you need to know the number of protons neutrons and electrons the element has In this section we ll show a sample Bohr diagram for hydrogen H Hydrogen 1 proton 1 electron 0 neutrons You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom
Sep 16 2022 0183 32 Bohr s model suggests that the atomic spectra of atoms is produced by electrons gaining energy from some source jumping up to a higher energy level then immediately dropping back to a lower energy level and emitting the energy difference between the two energy levels Lesson 1 Models of an atom Discovery of the electron and nucleus Rutherford s gold foil experiment Drawback of the Rutherford model Bohr s model of an atom Atomic structure Science gt Class 9 Chemistry India gt Structure of the Atom gt Models of an atom Bohr s model of an atom How did Bohr respond to the drawbacks in the Rutherford model
This page contains materials for the session on the atomic models of Rutherford and Bohr It features a 1 hour lecture video and also presents the prerequisites learning objectives reading assignment lecture slides homework with solutions and resources for further study