How To Merge Data From Multiple Excel Tabs Guidelines reflect a harmonised approach of the EU Member States and the Agency on how to interpret and apply the requirements for the demonstration of quality safety and efficacy set
Jun 26 2024 0183 32 Regulatory standards are paramount in pharmaceutical packaging Packaging must comply with guidelines set by regulatory bodies such as the Medicines and Healthcare Jan 1 2021 0183 32 The Medicines and Healthcare products Regulatory Agency MHRA is the UK s standalone medicines and medical devices regulator The below guidance should be followed
How To Merge Data From Multiple Excel Tabs
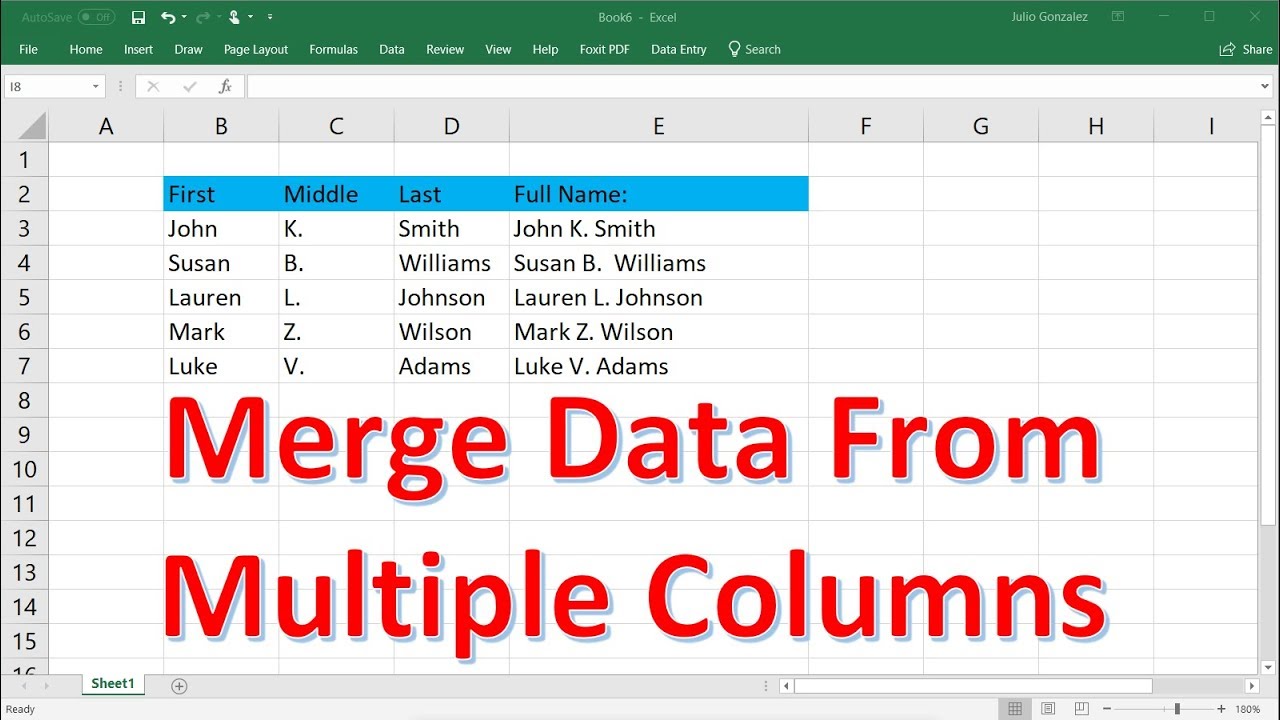 How To Merge Data From Multiple Excel Tabs
How To Merge Data From Multiple Excel Tabs
https://i.ytimg.com/vi/Pq5pHBDawv8/maxresdefault.jpg
The European Medicines Agency s Committee for Medicinal Products for Human Use prepares scientific guidelines in consultation with regulatory authorities in the European Union EU
Pre-crafted templates provide a time-saving service for developing a varied range of files and files. These pre-designed formats and layouts can be made use of for numerous personal and expert tasks, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, simplifying the content production procedure.
How To Merge Data From Multiple Excel Tabs

How To Merge Data From Multiple Workbooks In Excel 5 Methods
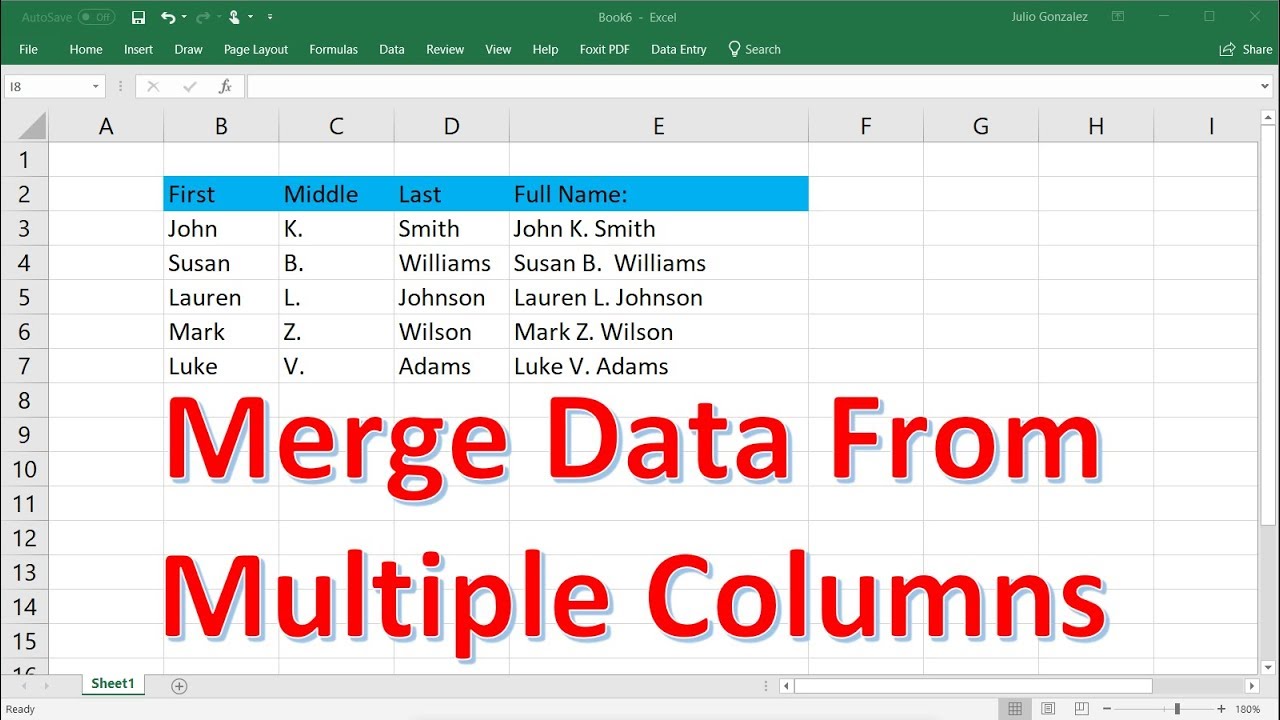
How To Merge Data In Excel Combine Multiple Sheets In Excel Using
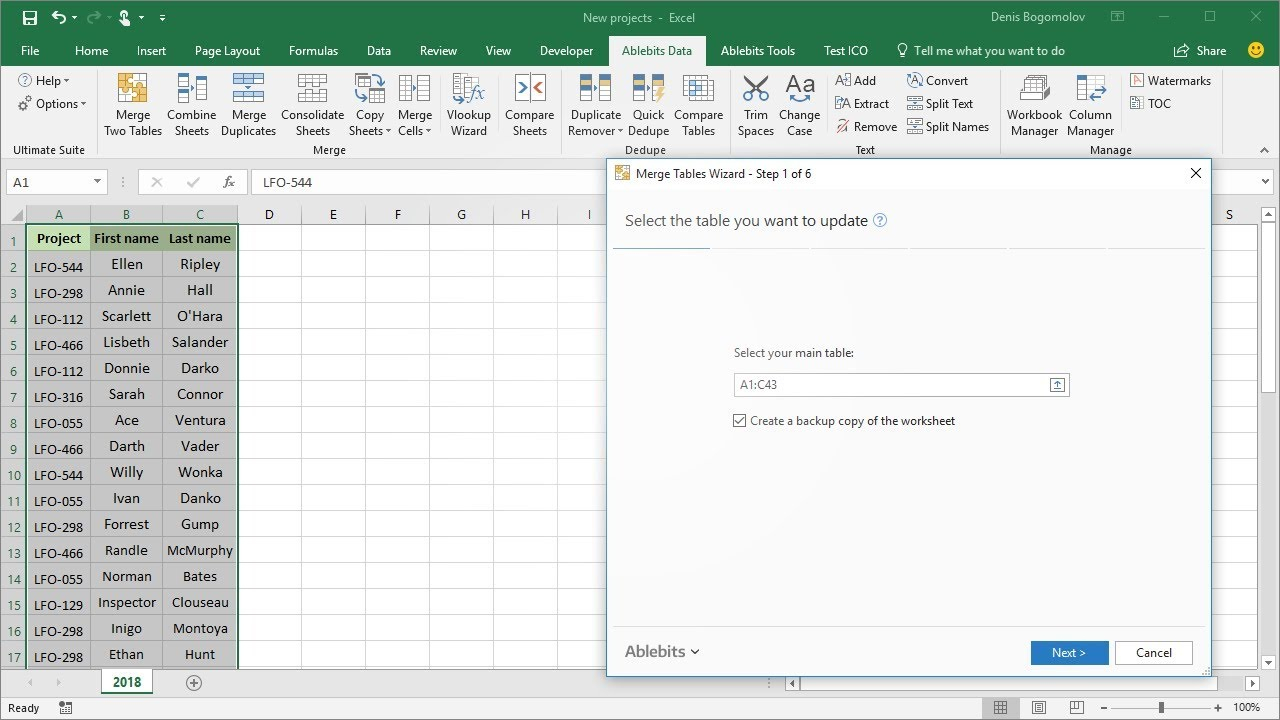
How To Merge Excel Spreadsheets Pertaining To Merge Multiple Worksheets
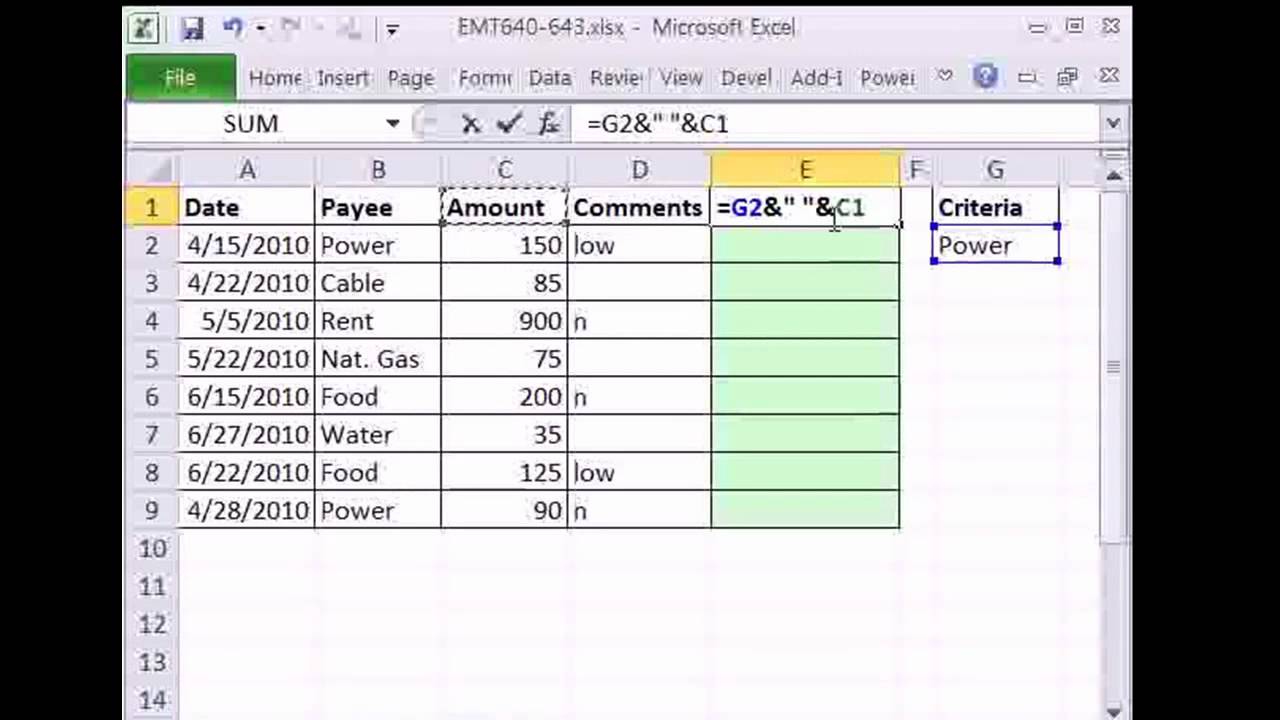
Pulling Data From Multiple Excel Files To A Single Excel File For Excel
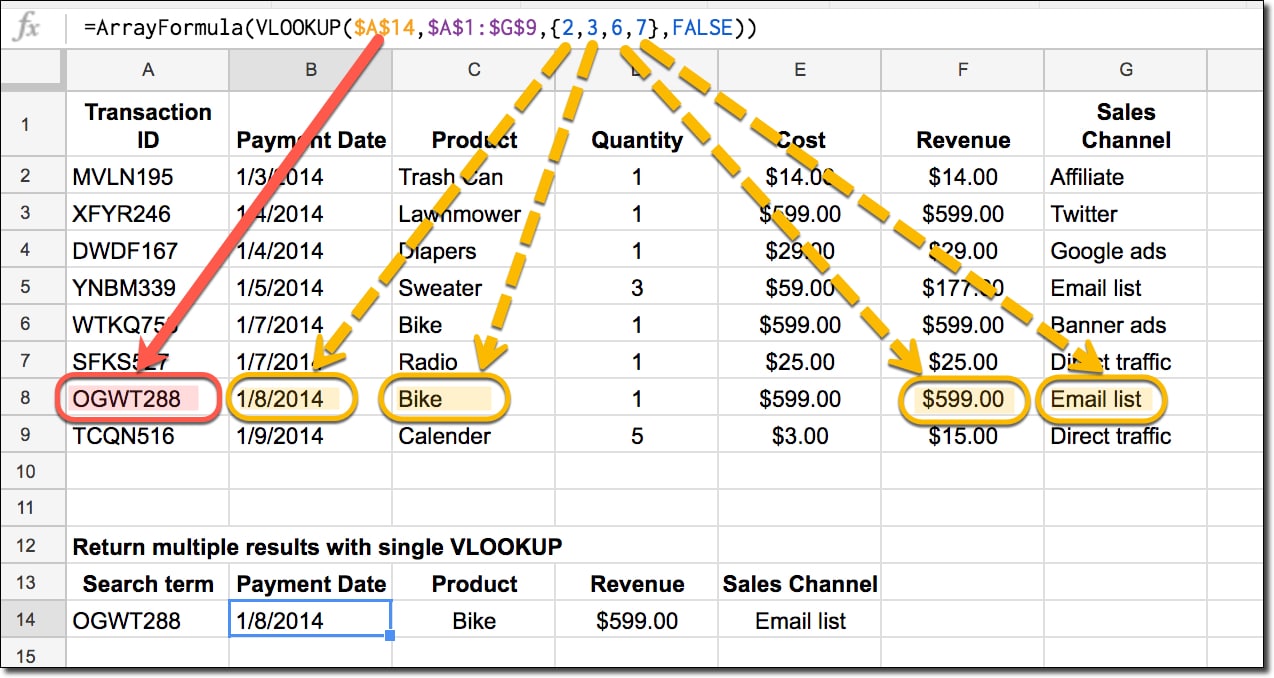
How To Merge Data In Excel Using Vlookup
How To Merge Excel Files Vrogue
https://www.gov.uk › government › publications ›
Mar 11 2025 0183 32 From 1 January 2025 in order to enable medicines to use the same packaging and labelling across the UK packaging for all UK medicinal products Prescription Only Medicine

https://www.originltd.com › useful-resources › safety
General pharma packaging rules regulations and standards The UK government offers guidance on the manufacturing and sale of pharma packaging designed to keep patients safe

https://dynascan.co.uk › a-guide-to-pharmaceutical
May 16 2025 0183 32 EMA EU The EMA requires adherence to European Pharmacopoeia standards ensuring that packaging maintains the integrity and identity of medicinal products MHRA UK
https://www.pharmagmp.in › gmp-for
Jan 27 2025 0183 32 MHRA operates independently in the UK particularly after Brexit Key differences include EMA guidelines are harmonized across the EU while MHRA may introduce UK

https://www.gov.uk › government › publications › best
Dec 29 2014 0183 32 Updated to reflect changes in regulations following Brexit transition This guidance explains the legal framework for labelling and packaging as described in UK legislation and
[desc-11] [desc-12]
[desc-13]