Ideal Gas Law Worksheet Pdf Express the temperature in degrees Celsius How many moles of chlorine gas would occupy a volume of 35 5 L at a pressure of 100 0 kPa and a temperature of 100
A sample of carbon monoxide at 57oC and under 0 67 atm of pressure takes up 85 3 L of space What mass of carbon monoxide is present in the sample Calculate the volume of this gas at a pressure of 2 3 atm and a temperature of 301 K 3 A 3 25 L container of ammonia gas exerts a pressure of 652 mm Hg at a
Ideal Gas Law Worksheet Pdf
 Ideal Gas Law Worksheet Pdf
Ideal Gas Law Worksheet Pdf
https://img.yumpu.com/35073400/1/500x640/ideal-gas-law-practice-worksheet.jpg
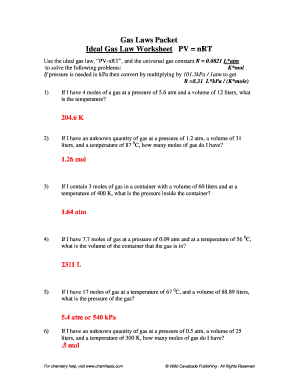
Ideal Gas Law Problems Use the ideal gas law to solve the following problems 1 If I have 4 moles of a gas at a pressure of 5 6 atm and a volume of 12
Templates are pre-designed documents or files that can be utilized for different purposes. They can conserve effort and time by offering a ready-made format and design for developing various kinds of content. Templates can be used for individual or expert projects, such as resumes, invites, flyers, newsletters, reports, presentations, and more.
Ideal Gas Law Worksheet Pdf

Ideal Gas Law Problems Worksheet - Fill Online, Printable, Fillable, Blank | pdfFiller

Ideal Gas Law II Worksheet by Scorton Creek Publishing - Kevin Cox

SCH3UAPS - Ideal Gas Law - South Pasadena Chemistry Name Period Date / / 12 The Gas Laws THE IDEAL GAS LAW PV = nRT where P = pressure in atmosphere V = | Course Hero

Gas law packet answers | PDF

Ideal Gas Law practice worksheet by MJ | TPT

The Ideal Gas Law and Dalton's Law -- Notes and Worksheet Set by Chemistry Wiz

https://uomus.edu.iq/img/lectures21/MUCLecture_2022_1226059.pdf
Ideal Gas Law Worksheet PV nRT Use the ideal gas law PerV nRT and the universal gas constant R 0 0821 L atm to solve the following problems K mol

http://misterguch.brinkster.net/WKS001_011_182581.pdf
Use the ideal gas law to solve the following problems 1 If I have 4 moles of a gas at a pressure of 5 6 atm and a volume of 12
https://www.csusm.edu/stemsc/worksheets/project1/chem150_ideal_gas_laws.pdf
Worksheet CHEM 150 Ch 10 Ideal Gas Law 1 How many moles of gas air are in the lungs of an adult with a lung capacity of 3 9 L Assume that the lungs

https://www.npsd.k12.nj.us/cms/lib04/NJ01001216/Centricity/Domain/474/Ch%2014%20Gas%20Laws%20Review%20WS.pdf
The Ideal and Combined Gas Laws PV nRT or P1V1 P2V2 T1 T2 Use your knowledge of the ideal and combined gas laws to solve the following problems

https://www.claytonschools.net/cms/lib/MO01000419/Centricity/Domain/206/Ideal%20Gas%20Law%20Worksheet%20KEY.pdf
IDEAL GAS LAW Name Key G Use the Ideal Gas Law below to solve the following problems PV nRT where P pressure in atmospheres V volume in liters n
If I have 4 moles of a gas at a pressure of 5 6 atm and a volume of 12 liters what is the temperature PV ART The KR TE PV hR tatat 205K or T 200 K Concepts This worksheet gives students practice using the ideal gas law PV nRT Students will find pressure P volume V temperature T
Mixed Gas Laws Worksheet Solutions 1 How many moles of gas occupy 98 L at a pressure of 2 8 atmospheres and a temperature of 292 K n PV 2 8 atm 98