Isotopes And Atomic Mass Worksheet Uranium has three common isotopes If the abundance of 254U is 0 01 the abundance of 235U is 0 71 and the abundance of 238U is 99 28 what is
How can you tell one isotope from another Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element Isotopes and Atomic Mass Isotopes Mixtures Isotopes and Atomic Mass Isotopes Mixtures m
Isotopes And Atomic Mass Worksheet
 Isotopes And Atomic Mass Worksheet
Isotopes And Atomic Mass Worksheet
https://ecdn.teacherspayteachers.com/thumbitem/Dollar-Practice-Set-Isotopes-and-Average-Atomic-Mass-8350954-1659094520/original-8350954-4.jpg
Atomic number Atomic Mass and Isotopes Worksheet and PowerPoint Created by Classroom Chemist No prep complete lesson to teach
Templates are pre-designed files or files that can be utilized for various functions. They can save effort and time by providing a ready-made format and layout for developing various sort of material. Templates can be utilized for individual or professional tasks, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
Isotopes And Atomic Mass Worksheet
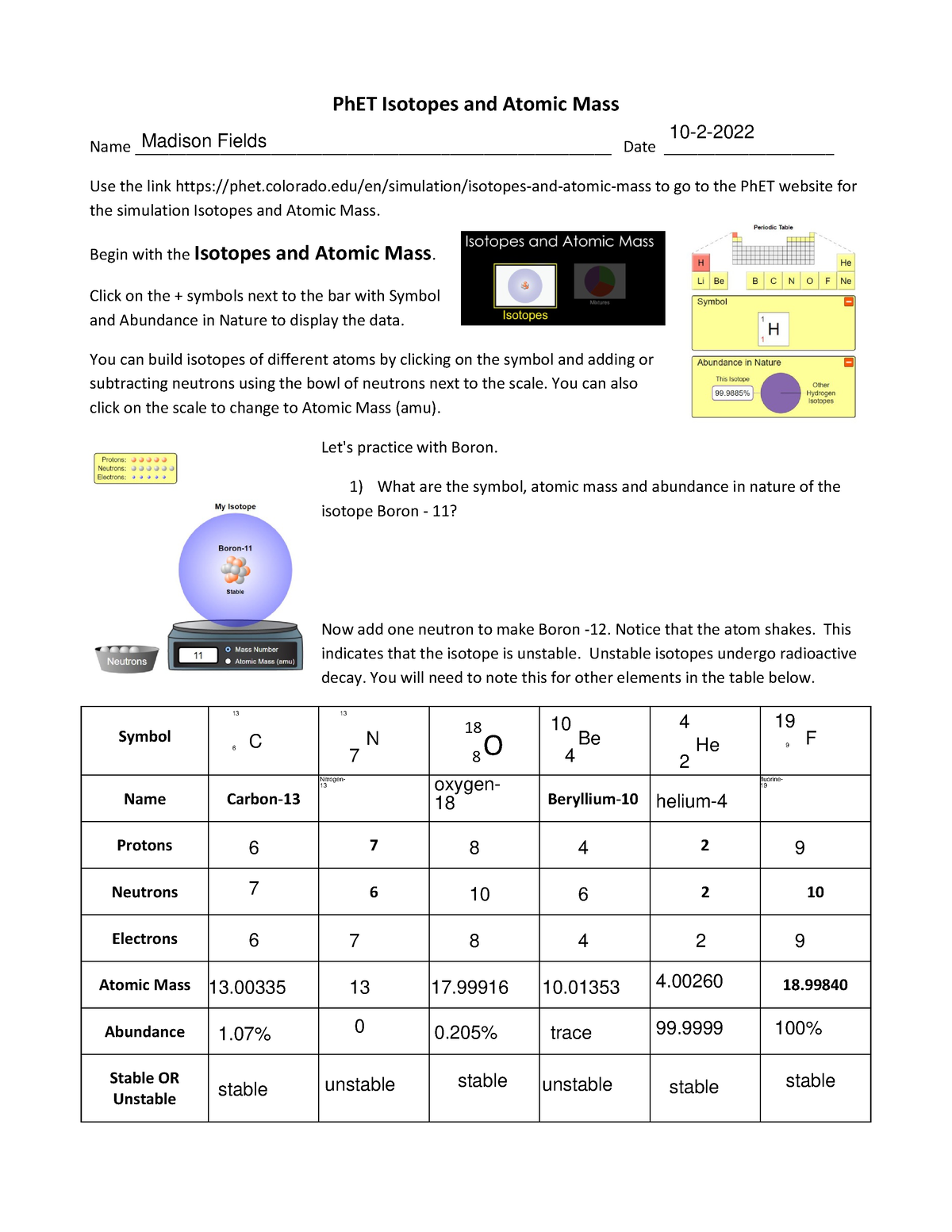
Ph ET Isotopes WS - Its just a worksheet - 18 8 PhET Isotopes and Atomic Mass Name - Studocu

Average Atomic Mass and Isotopes: Exploring Carbon, Isotope | Course Hero

Chemistry Isotopes & Atomic Mass Guided Inquiry Lesson | TPT

Practice - Average Atomic Mass Worksheet 2.0 by The Chem Teacher
![Isotopes and Atomic Mass - What particles determine the mass number? [1] The neutrons and protons - Studocu isotopes-and-atomic-mass-what-particles-determine-the-mass-number-1-the-neutrons-and-protons-studocu](https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/bf5f1d406ed5a50ba391c96c9b2f6f8b/thumb_1200_1553.png)
Isotopes and Atomic Mass - What particles determine the mass number? [1] The neutrons and protons - Studocu

SOLUTION: Week 6 Laboratory Phet Assignment - Studypool

https://www.mayfieldschools.org/Downloads/Atmoic%20%20mass%20Isotopes%20and%20Stuff%20and%20KEY2.pdf
Complete the following chart Isotope name atomic mass of protons of neutrons of electrons Uranium 235 Chlorine 35 Calcium 48 Strontium 90
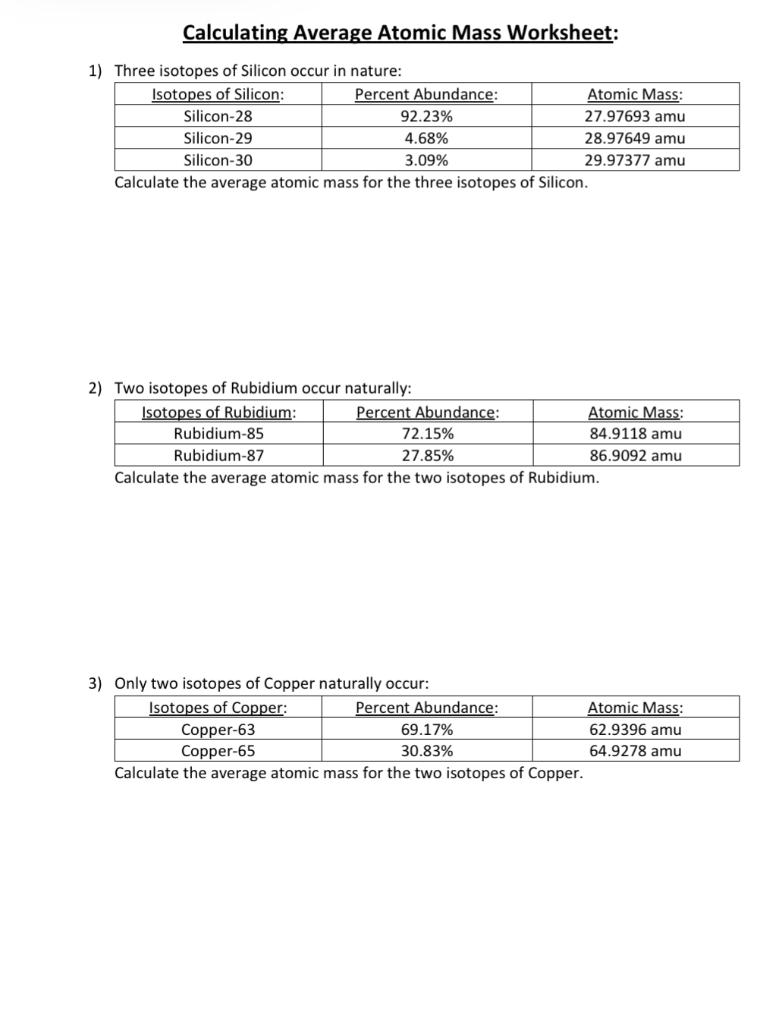
https://www.cbsd.org/cms/lib/PA01916442/Centricity/Domain/2209/isotope%20practice.doc
Calculate the atomic mass of copper if copper 63 is 69 17 abundant and copper 65 is 30 83 abundant 7 Boron exists in two isotopes boron 10 and boron 11

https://www.studocu.com/row/document/stmarys-catholic-high-school/chemistry/ph-et-isotopes-ws-its-just-a-worksheet/34681385
Its just a worksheet phet isotopes and atomic mass madison fields name date use the link to go to the phet website for the simulation isotopes

http://www.nyostrander.us/Chemistry/AnswerKeys/1Y1IsotopesAtomicMassWSKey.pdf
Isotopes and Average Atomic Mass Isotopes are atoms of the same element they have the same number of protons but with different

https://ca01001129.schoolwires.net/cms/lib/CA01001129/Centricity/Domain/1890/W.isotope%20practice%20and%20KEY.doc
Isotope Practice Worksheet Name LT 3 1 Use the periodic table to identify and count subatomic particles within the atom I
Atomic mass of rubidium 85 56 amu 2 Uranium has three common isotopes If the abundance of 234U is 0 01 1 The element copper has naturally occurring isotopes with mass numbers of 63 and 65 The relative abundance and atomic masses are 69 2 and 30 8 respectively
Yahoo Answers Answers about Atomic Mass Phet Isotopes And Atomic Mass Worksheet Answer Key Isotopes Percent Abundance Atomic Mass How to Pass