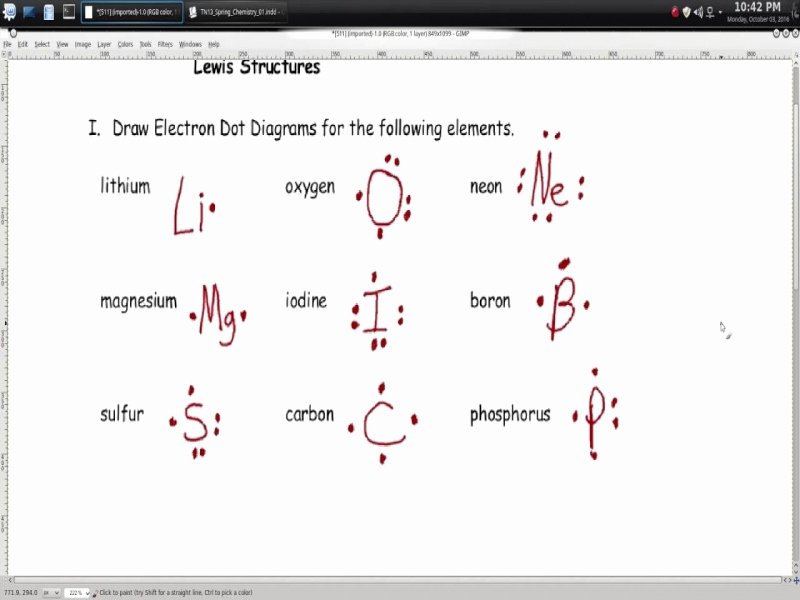
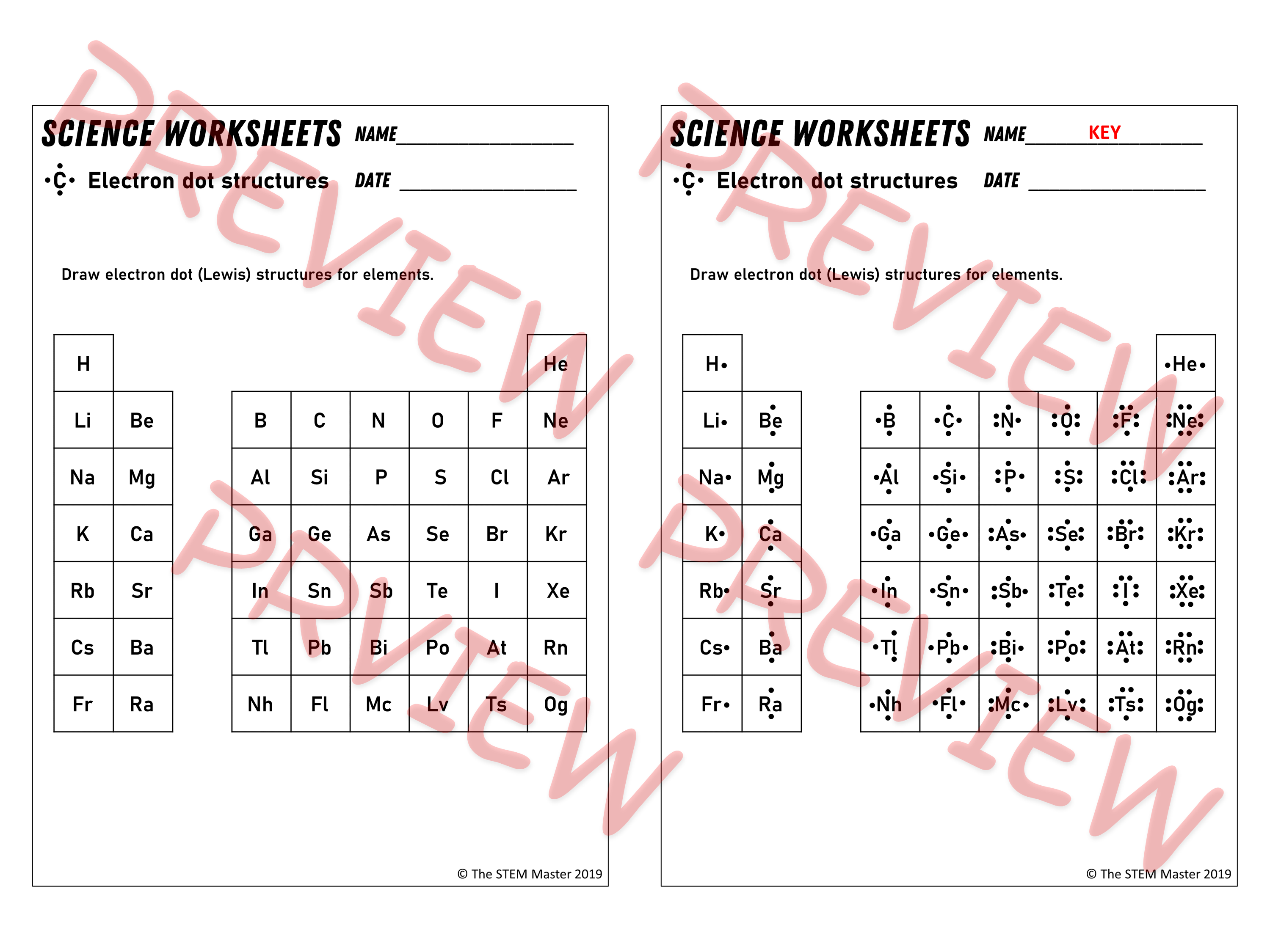
Lewis Dot Diagram Worksheet Grade 10 This customizable and printable worksheet is designed for students to practice drawing Lewis dot diagrams of various chemical elements You can choose the number of problems and range of elements to include on each worksheet The worksheet is randomly generated from your preferences
07 26 Lewis Diagrams Made Easy How to Draw Lewis Dot Structures ketzbook 358 1 Select textbook and university Improve your experience by picking them 1 Intro to General Chemistry 3h 53m This Lewis Structures Worksheet is suitable for 10th 11th Grade In this Lewis structures activity students identify and describe what Lewis structures or dot diagrams are and how they illustrate the valence electrons in an outer shell Then they use a periodic table to determine the numbers of valence electrons for each of the 20 elements
Lewis Dot Diagram Worksheet Grade 10
 Lewis Dot Diagram Worksheet Grade 10
Lewis Dot Diagram Worksheet Grade 10
https://stemsheets.com/img/5/og/lewis-dot-diagram-worksheet.png
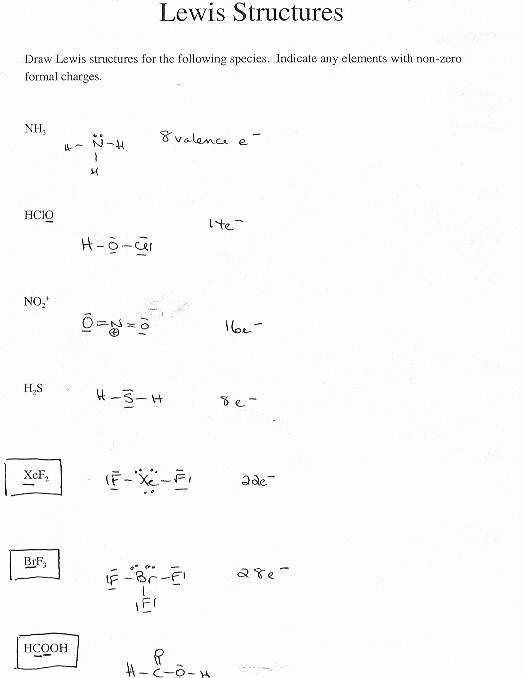
Lewis Structures Homework Draw the Lewis structures for the following compounds Make certain that All structures follow the octet rule There are the correct number of valence electrons All atoms have their correct charge PI3 N2 H2O AsBr3 SiCl4 8 HNO 9 AsN 10 CH3NH2 Solutions to Lewis Structures Homework 8 HNO 9 AsN 10 CH3NH2
Templates are pre-designed files or files that can be utilized for numerous functions. They can conserve effort and time by offering a ready-made format and design for creating different kinds of content. Templates can be used for individual or professional jobs, such as resumes, invites, flyers, newsletters, reports, discussions, and more.
Lewis Dot Diagram Worksheet Grade 10
46 Lewis Dot Diagram Worksheet

46 Lewis Dot Diagram Worksheet

Lewis Dot Diagram Worksheet In 2023 Educational Worksheets Practices
Lewis Dot Structures Worksheet

Chemistry Worksheet Lewis Dot Structures Answers Promotiontablecovers

46 Lewis Dot Diagram Worksheet

https://www.lcps.org/cms/lib/VA01000195/Centricity
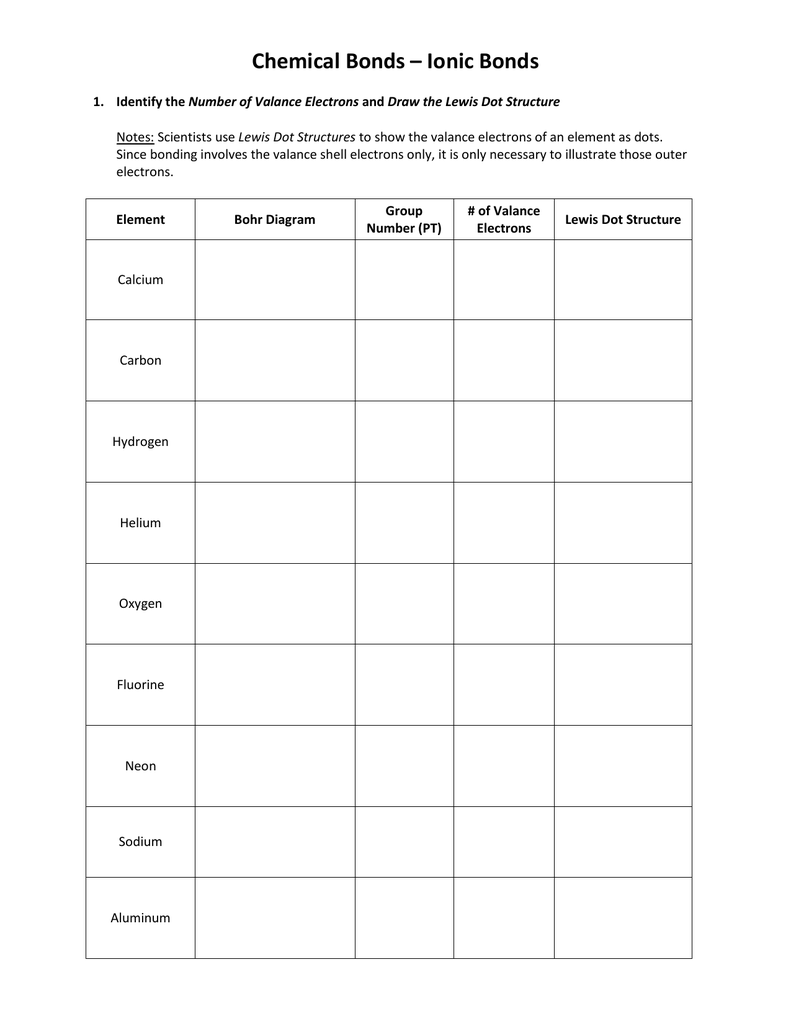
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons Once you have found the number of valance electrons place them around the elements symbol Element Atomic Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon Hydrogen Lithium Magnesium Boron
https://web.gccaz.edu//Lewispractice.pdf
Draw the Lewis dot structures for each of the following molecules a H2S c SO3 b CH2Br2 d HCN 3 Draw the Lewis dot structure for each of the following polyatomic ions a NH4 c PO4 3 b NO3 d CO3 2 4 For the following molecules or ions where the central atom is underlined Draw the Electron dot structure
https://chem.libretexts.org/Courses/University_of
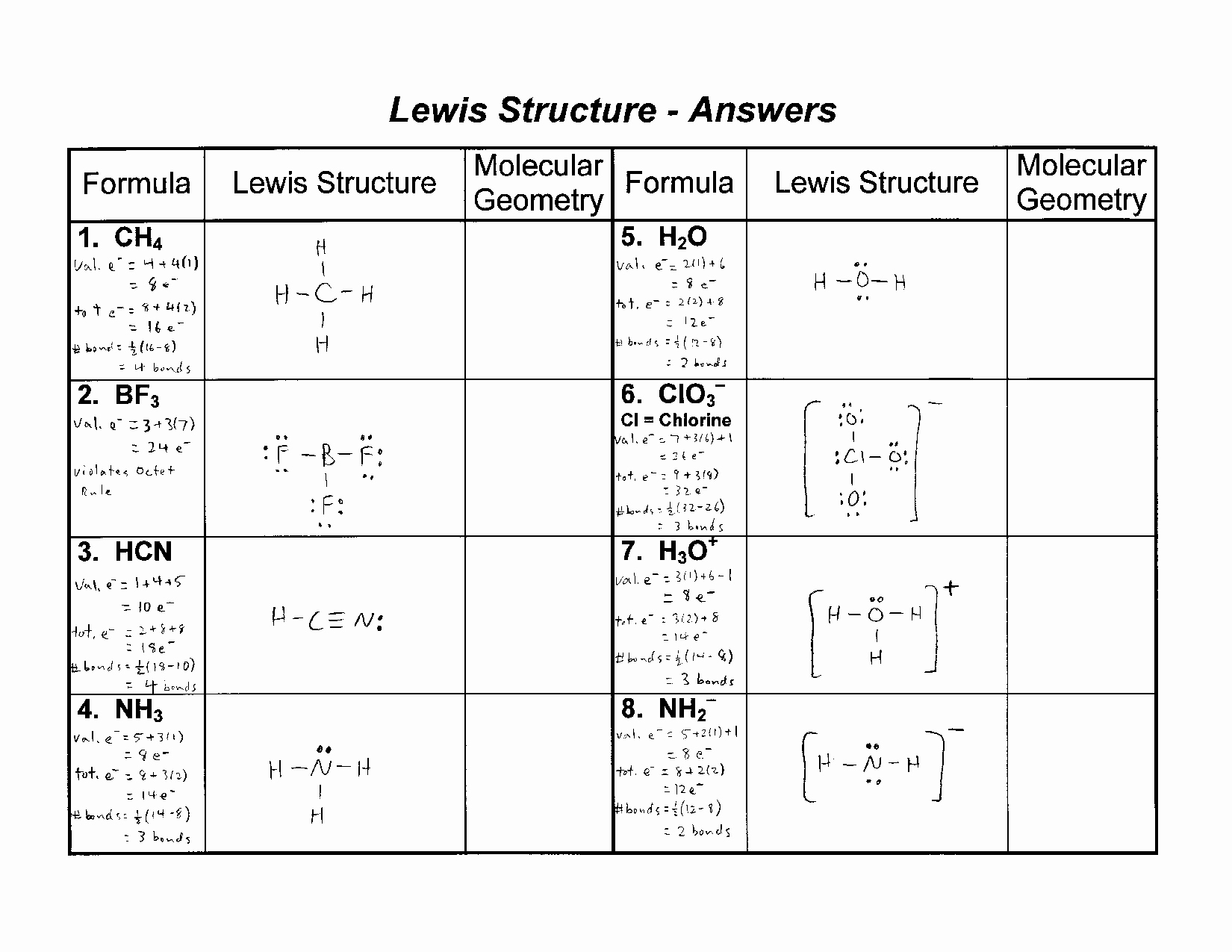
For each of the following draw the Lewis dot structure give the electron arrangement E A and the molecular geometry M G
https://chem.libretexts.org/Courses/College_of
May 20 2018 0183 32 The Lewis electron dot diagram for NO is as follows Although the O atom has an octet of electrons the N atom has only seven electrons in its valence shell Although NO is a stable compound it is very chemically reactive as are most other odd electron compounds Electron deficient molecules represent the second violation to the octet rule
/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg?w=186)
https://www.khanacademy.org//e/lewis-diagrams
Lewis diagrams Science gt AP 174 College Chemistry gt Molecular and ionic compound structure and properties gt Lewis diagrams Terms of use Lewis diagrams Google Classroom You might need Periodic table Ethanethiol C A 2
This is a one page worksheet covering the Lewis Dot Structure Students are provided with a Periodic Table of Elements and are tasked with filling in the valance electrons for a variety of elements The zip file includes two separate PDF versions A teacher s version with an answer key and a student version This digital or printable worksheet reviews identifying ionic and covalent bonds and compounds their elements and Lewis Dot Diagrams showing how the valence electrons are configured HS PS1 and MS PS1 2
For each of the following draw the Lewis dot structure give the electron arrangement E A and the molecular geometry M G