Limiting And Excess Reactants And Percent Yield Worksheet ID 758816 25 02 2021 Country code AE Country United Arab Emirates School subject Chemistry 1061818 Main content Stoichiometry 2009395 Practice the calculations to find the limiting reagents and yields Other contents Limiting reactants and
Explain the concepts of theoretical yield and limiting reactants reagents Derive the theoretical yield for a reaction under specified conditions Calculate the percent yield for a reaction Limiting Reagent and Percent Yield Worksheet Name Period 1 When copper II chloride reacts with sodium nitrate copper II nitrate and sodium chloride are formed The balanced chemical equation is CuCl2 2 NaNO3 Cu NO3 2 2 NaCl a
Limiting And Excess Reactants And Percent Yield Worksheet
 Limiting And Excess Reactants And Percent Yield Worksheet
Limiting And Excess Reactants And Percent Yield Worksheet
https://s3.studylib.net/store/data/025262652_1-f29aa5b335135028d153f5b57ca8edb7.png
Course Chemistry library gt Unit 5 Lesson 3 Limiting reagent stoichiometry Limiting reactant and reaction yields Worked example Calculating the amount of product formed from a limiting reactant Introduction to gravimetric analysis Volatilization gravimetry Gravimetric analysis and precipitation gravimetry
Templates are pre-designed files or files that can be used for different purposes. They can save effort and time by offering a ready-made format and design for creating different type of material. Templates can be utilized for personal or professional tasks, such as resumes, invites, flyers, newsletters, reports, discussions, and more.
Limiting And Excess Reactants And Percent Yield Worksheet

Limiting Reactant Excess Reactant Percent Yield And Percent Purity

How To Determine Limiting And Excess Reactants Using Theoretical Yield
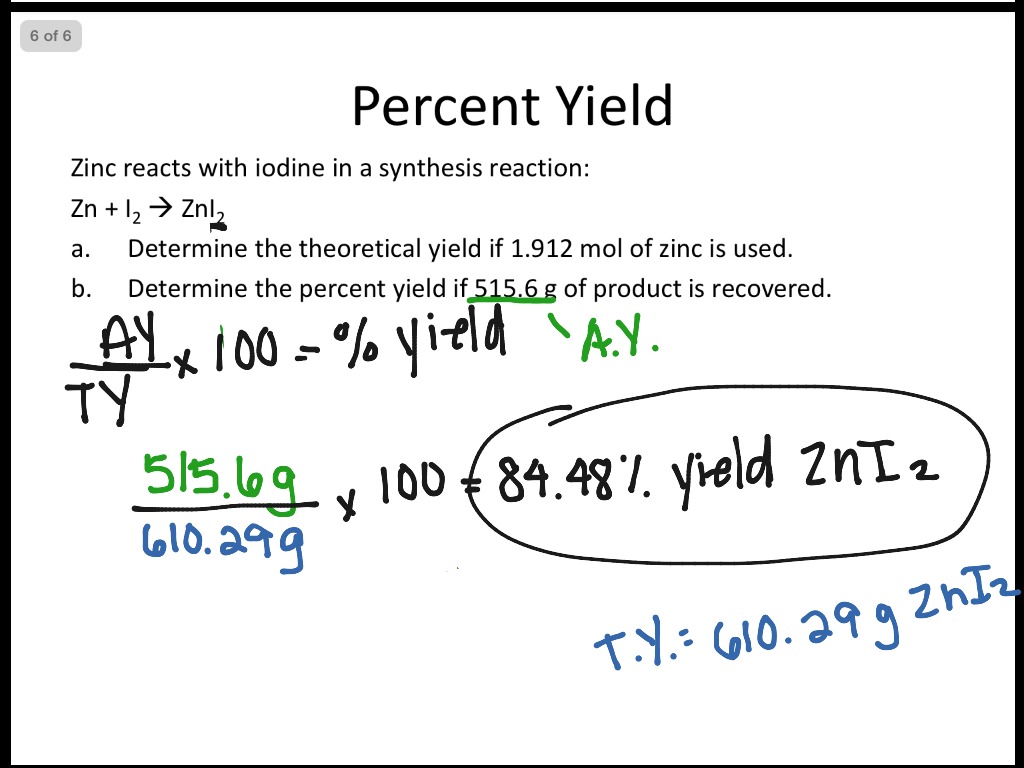
Percent Yield Worksheet
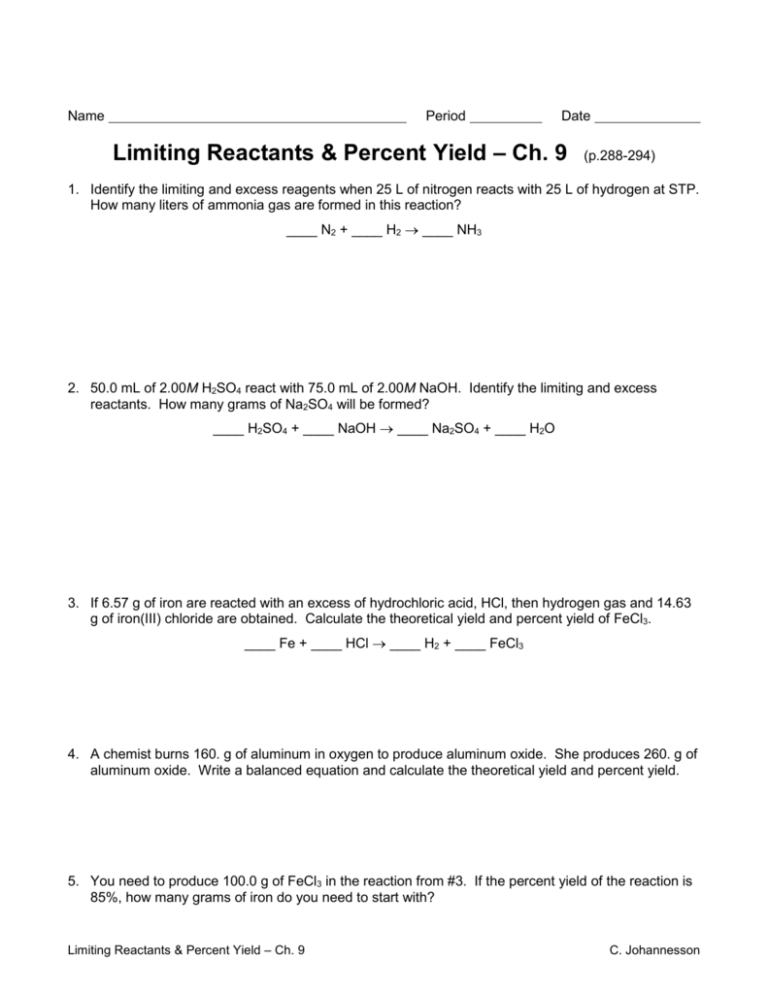
Limiting And Excess Reactants Worksheet Db excel

Limiting Reactant Worksheet
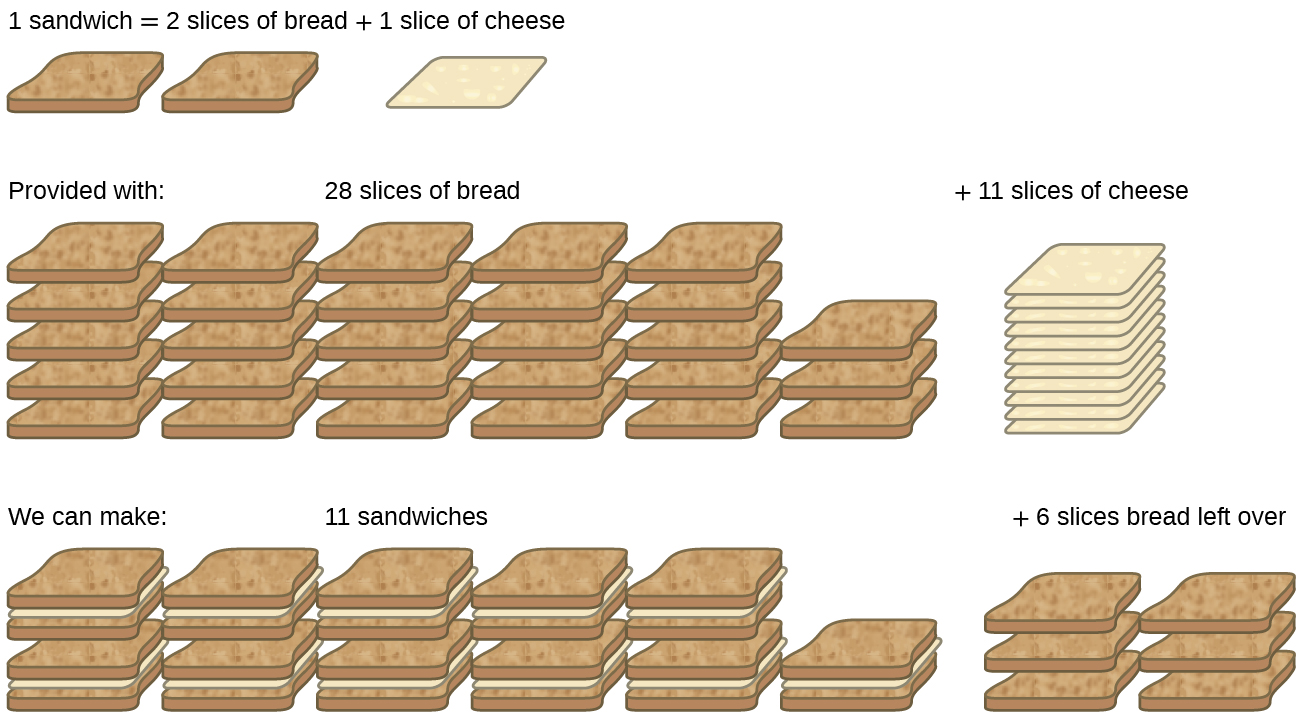
5 4 Limiting Reactant And Reaction Yields Introduction To Chemistry

https://scienceatcchs.weebly.com/uploads/3/7/8/4/
1 Consider the following reaction NH4NO3 Na3PO4 NH4 3PO4 NaNO3 Which reactant is limiting assuming we started with 30 0 grams of ammonium nitrate and 50 0 grams of sodium phosphate What is the mass of each product that can be formed What mass of the excess reactant s is left over 2 Consider the following reaction

https://chem.libretexts.org/Bookshelves/General
Aug 14 2020 0183 32 Limiting Reactants in Solutions The concept of limiting reactants applies to reactions carried out in solution as well as to reactions involving pure substances If all the reactants but one are present in excess then the amount of the limiting reactant may be calculated as illustrated in Example PageIndex 2
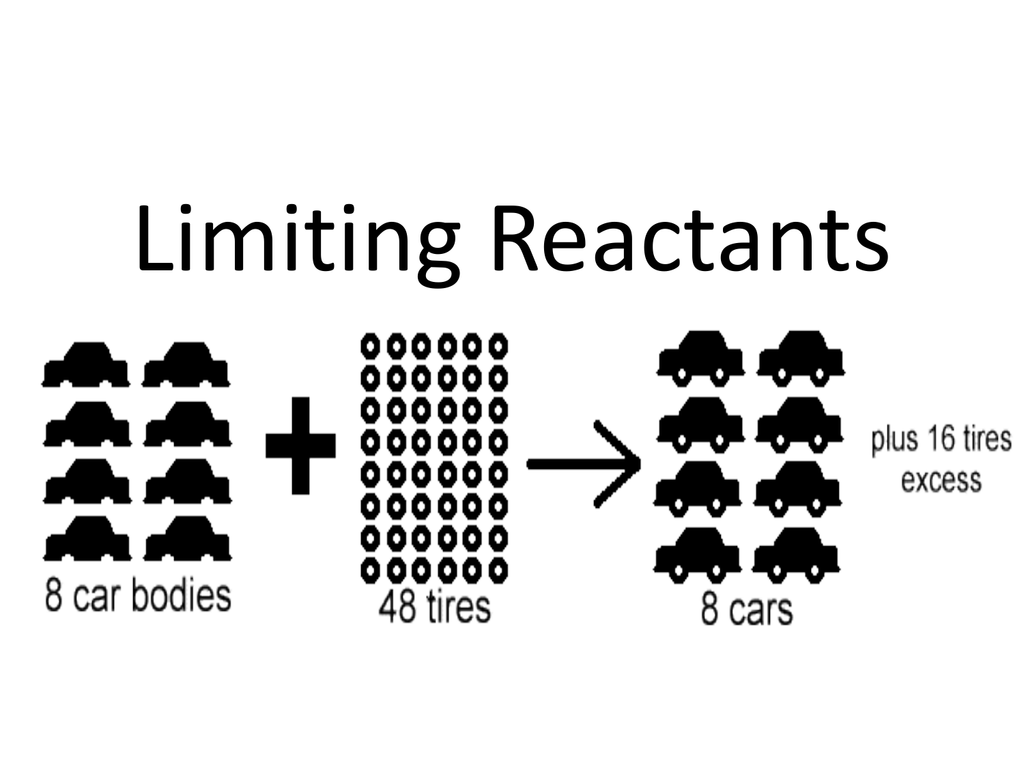
https://chem.libretexts.org/Courses/Anoka-Ramsey
Apr 8 2023 0183 32 Identify the limiting reactant s and excess reactant s The limiting reactant is Rb since it would yield the least amount of product 0 711 g Mg The excess reactant is MgCl 2 since its complete reaction would have yielded up to 0 878 g Mg Calculate the mass of excess reactant that reacts

http://www.simplychemistry.org/chemistry/Pearson Chemistry
Limiting and Excess Reagents How is the amount of product in a reaction affected by an insufficient quantity of any of the reactants Many cooks follow a recipe when making a new dish They know that suffi cient quantities of all the ingredients must be available in order to follow the Key Questions How is the amount of recipe

https://www.ahschools.us/cms/lib/MN01909485
Microsoft Word Limiting Reactant and Percent Yield Worksheet new 3 15 Honors Chemistry 1B Limit Reactant and Percent Yield Worksheet with excess calculation Name 1 Balance the equation for the reaction given below CuCl2 NaNO3 Cu NO3 2 NaCl
2 50 Zip Students will gain so much value from these doodle notes that they will be proud of and use The included teacher notes suggest many ways of incorporating doodle notes in your class and includes an example suggestion for completing the doodle notes For interactive notebooks just print at 90 and it will fit beautifully 3 based on the moles that you have calculate the moles that you need of the other reagent to react with each of those amounts 4 compare what you have to what you need If you have more than you need this is the reagent in excess xs If you have less than you need this is the limiting reagent LR
Method 1 For the first method we ll determine the limiting reactant by comparing the mole ratio between Al and Cl A 2 in the balanced equation to the mole ratio actually present In this case the mole ratio of Al and Cl A 2 required by balanced equation is moles of Al moles of Cl 2 required 2 3 0 6 6