Limiting And Excess Reactants Worksheet Answers Race Car PED CHEMISTRY 09 0901 012 L3 indd Key Objectives 12 3 1 EXPLAIN how the amount of product in a reaction is affected by an insufficient quantity of any of the reactants 12 3 2 EXPLAIN what the percent yield of a reaction measures Additional Resources
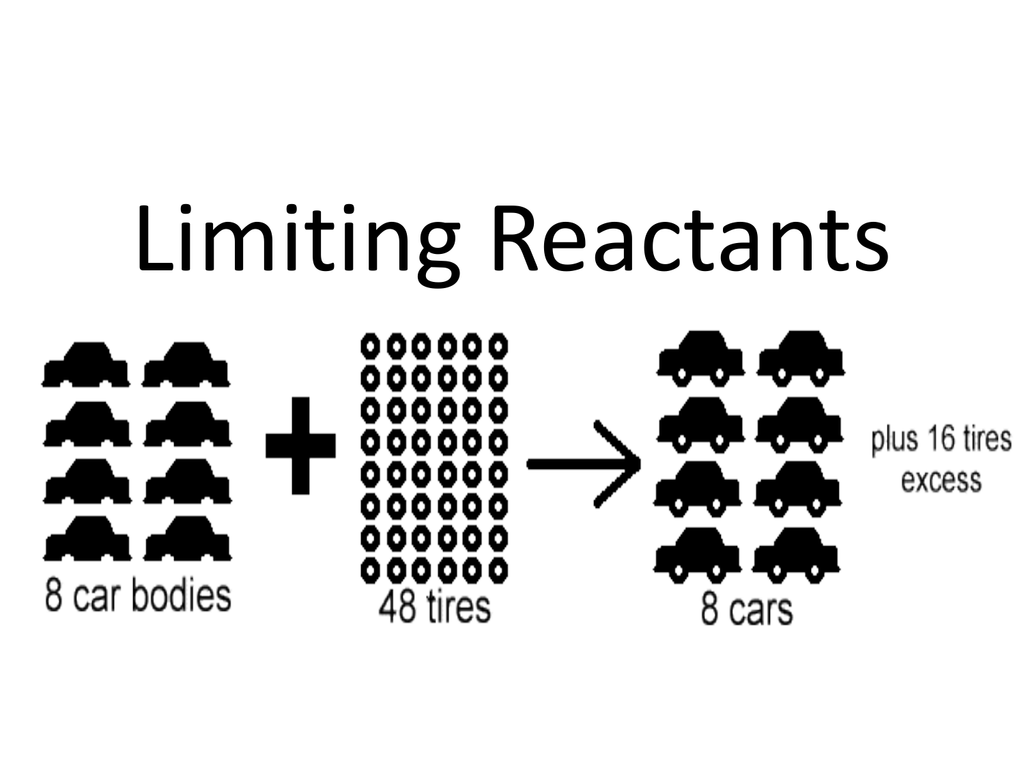
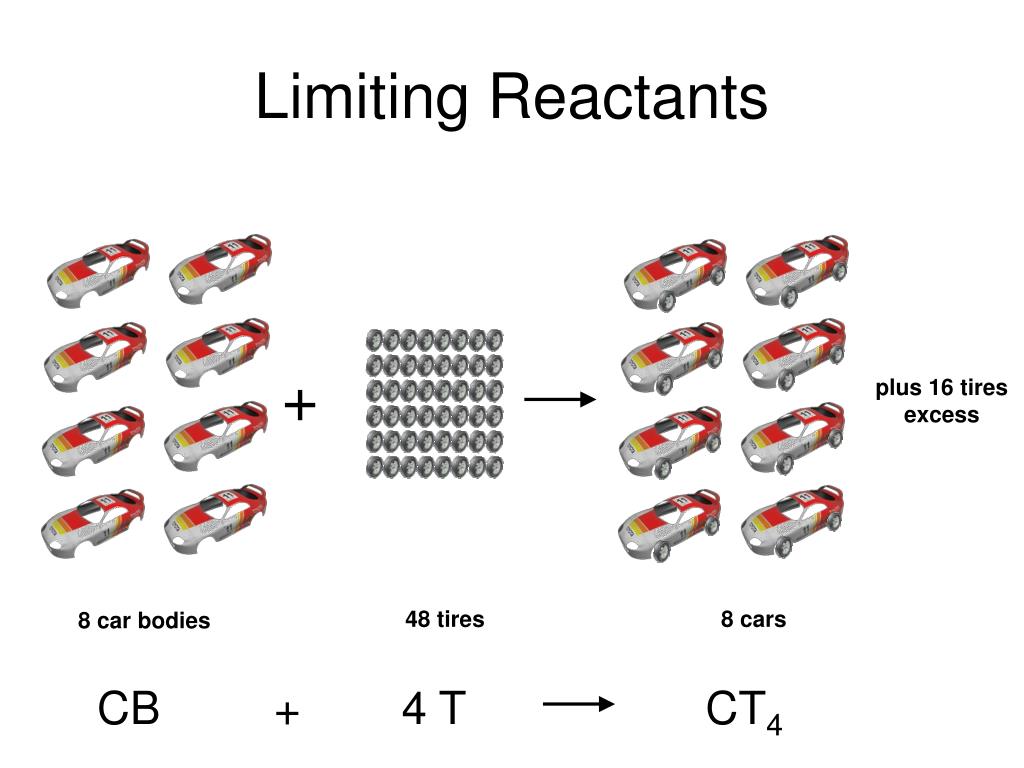
Limiting and Excess Reactants Is there enough of each chemical reactant to make a desired amount of product Why If a factory runs out of tires while manufacturing cars production stops No more cars can be fully built without ordering more tires A similar thing happens in a chemical reaction To determine the amounts of product either grams or moles you must start with the limiting reagent Use the amount that you have not the amount you need To determine the grams of excess reagent subtract the amount you need from the amount that you have then using the molar mass convert the moles left to grams
Limiting And Excess Reactants Worksheet Answers Race Car
 Limiting And Excess Reactants Worksheet Answers Race Car
Limiting And Excess Reactants Worksheet Answers Race Car
https://static.docsity.com/documents_first_pages/2022/08/05/c0eb3fb008b305b3068a805409eddc5c.png?v=1671195856
Course Chemistry library gt Unit 5 Lesson 3 Limiting reagent stoichiometry Limiting reactant and reaction yields Worked example Calculating the amount of product formed from a limiting reactant Introduction to gravimetric analysis Volatilization gravimetry Gravimetric analysis and precipitation gravimetry
Pre-crafted templates use a time-saving solution for developing a diverse variety of files and files. These pre-designed formats and layouts can be utilized for various individual and professional tasks, including resumes, invites, flyers, newsletters, reports, presentations, and more, improving the content creation procedure.
Limiting And Excess Reactants Worksheet Answers Race Car

SOLUTION Stoichiometry And Limiting Reactant Lab Studypool

Limiting And Excess Reactants Worksheet Answers Race Car Printable

Free Printable Limiting Reagent Worksheets
Limiting Reactants

Limiting And Excess Reactants Worksheet Answers Pdf Study Finder
PPT Limiting Reactants PowerPoint Presentation Free Download ID

https://msdemonte.weebly.com/uploads/3/0/5/6/
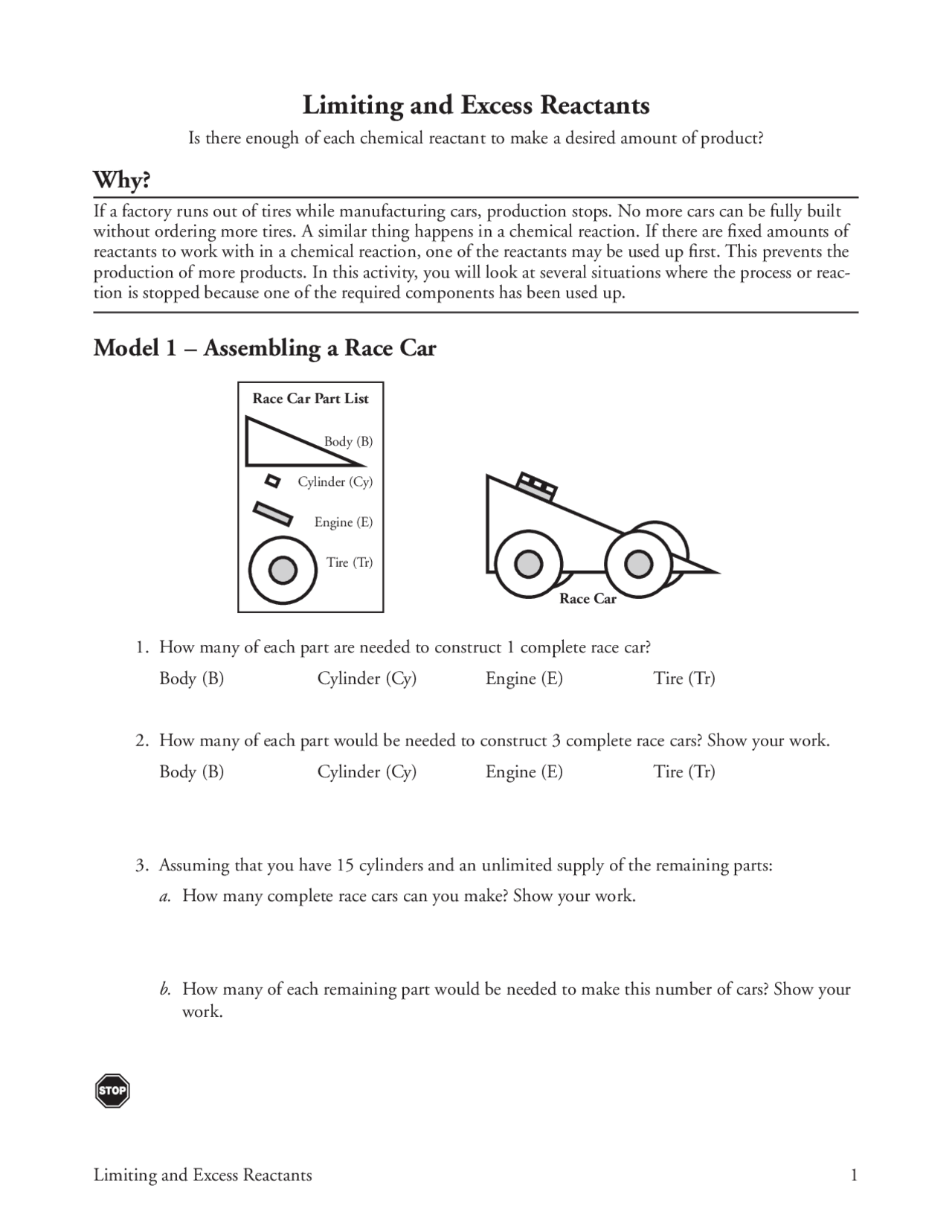
Limiting and Excess Reactants of to a out of while No more A Ching a If with in of the br up This p In will Model I Assembling a Race Car of are to complete 2 3 Show 3 15 of H many 5 3 ing bc to IS Model 2 Manufacturing Race Cars

http://pantherchemistry.weebly.com/uploads/1/9/0/4/
Model 1 1 Answer the following questions about the race car toy represented in Model 1 a How many bodies are needed to make one race car b How many cylinders are needed to make one race car c How many tires are needed to make one race car d How many engines are needed to make one race car Model 2 2
http://www.morrischem.weebly.com/uploads/8/2/4/7/82476834/
Limiting and Excess Reactants Is there enough of each chemical reactant to make a desired amount of product Why If a factory runs out of tires while manufacturing cars production stops No more cars can be fully built without ordering more tires A similar thing happens in a chemical reaction

https://www.mcmsnj.net/cms/lib07/NJ01911694
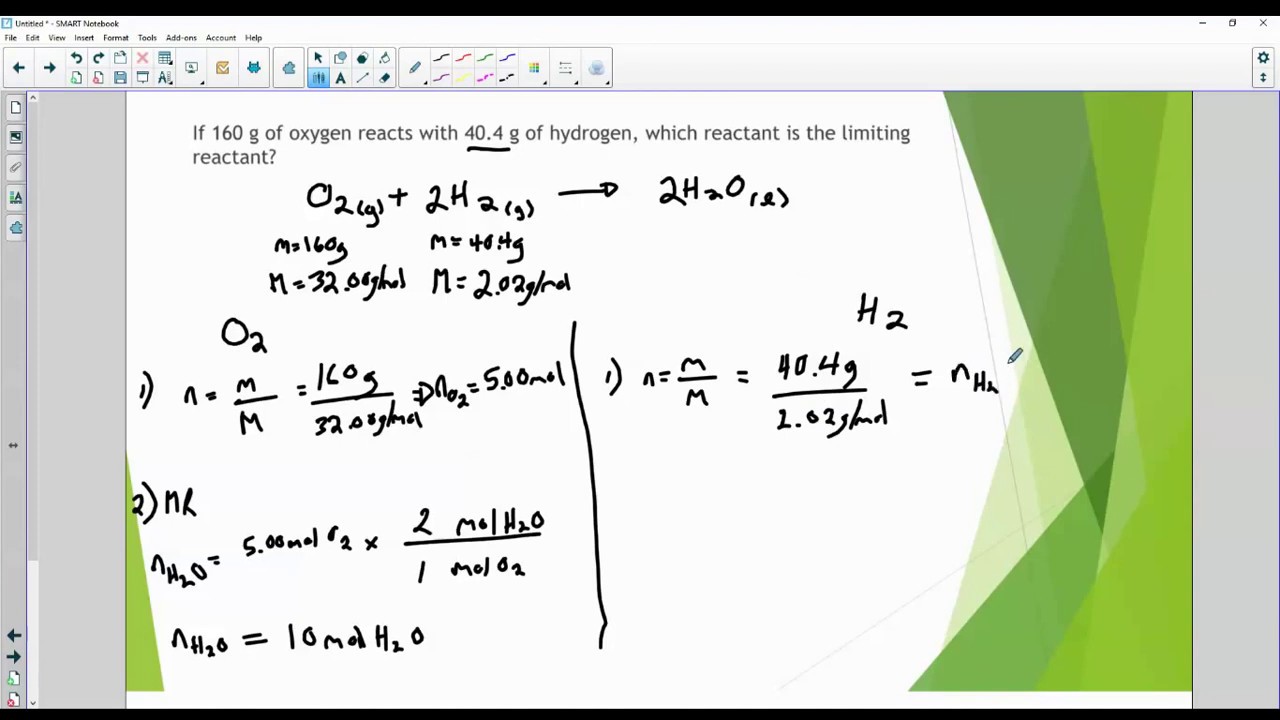
What is the limiting reactant How much excess is left over Can use either of the following to determine the limiting reactant Determine how much one of the reactant needs of the other 15 3 quot 1 1 quot quot 58 443 quot 1 quot quot quot 2 quot quot 331 208 quot quot 1 quot quot quot 43 4 quot quot 60 8 quot quot 1 1

https://chem.libretexts.org/Courses/Anoka-Ramsey
Identifying the limiting and excess reactants for a given situation requires computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation For example imagine combining 3 moles of H 2 and 2 moles of Cl 2
Jun 2 2020 0183 32 Limiting Reactants in Solutions The concept of limiting reactants applies to reactions carried out in solution as well as to reactions involving pure substances If all the reactants but one are present in excess then the amount of the limiting reactant may be calculated as illustrated in Example PageIndex 2 The equation for this reaction is Ca3 PO4 2 s 3H2SO4 aq 3CaSO4 s 2H3PO4 aq Determine the masses of both reactants calcium phosphate and sulfuric acid that would be required to produce 100 kg of calcium sulfate assuming the process is only 75 efficient Pearson Education Australia a division of Pearson Australia Group Pty Ltd 2008
The limiting reactant is MoO 3 since it produced fewer moles of ZnO Correct answer MoO 3 is the limiting reactant 6 In the reaction below 2 34 g I 2O 5 react with 3 40 g CO How many grams of CO 2 are produced I 2O 5 5 CO 224 5 CO 2 I 2 2 34 g I 2O 5 1 mol I 2O 5 5 mol CO 2 44 01 g CO 2 1 54 g CO 2 333 80 g I 2O 5 1 mol I 2O 5 1 mol