Mixed Gas Laws Practice Problems Answers For the rest of the problems First identify each number with P V or T Second state whose law you are using Third show the equation Fourth solve the problem and Fifth circle your final answer and make sure you don t forget your units 1 The gas in a sealed can is at a pressure of 3 00 atm at 250C A warning on the can tells the
Do the following problems showing your work and including all proper units 1 At 350oC nitrogen has a velocity of 800 m s Find the velocity of hydrogen at the same temperature 2 At room temperature acetylene C2H2 has a velocity of 480 m s At the same temperature an unknown noble gas has a velocity of 267 m s What is the unknown gas 3 1 The gas in a sealed can is at a pressure of 3 00 atm at 25 C A warning on the can tells the user not to store the can in a place where the temperature will exceed 52 C What would the gas pressure in the can be at 52 C 2 A sample of hydrogen exerts a pressure of 0 329 atm at 47 C The gas is heated 77 C at constant volume
Mixed Gas Laws Practice Problems Answers
 Mixed Gas Laws Practice Problems Answers
Mixed Gas Laws Practice Problems Answers
https://i.pinimg.com/736x/dd/3f/e2/dd3fe291b899e762c57e69a134b45bf0.jpg
The document provides 9 practice problems involving the ideal gas law Dalton s law of partial pressures and Graham s law of effusion The problems calculate various gas properties including pressure volume moles grams temperature and molar
Templates are pre-designed documents or files that can be used for numerous functions. They can save effort and time by supplying a ready-made format and design for creating various sort of content. Templates can be utilized for personal or professional projects, such as resumes, invites, flyers, newsletters, reports, presentations, and more.
Mixed Gas Laws Practice Problems Answers

SOLUTION College General Chemistry Gas Laws Tutorial And Answer Key

Worksheet Gas Laws

Avogadro s Law Worksheet
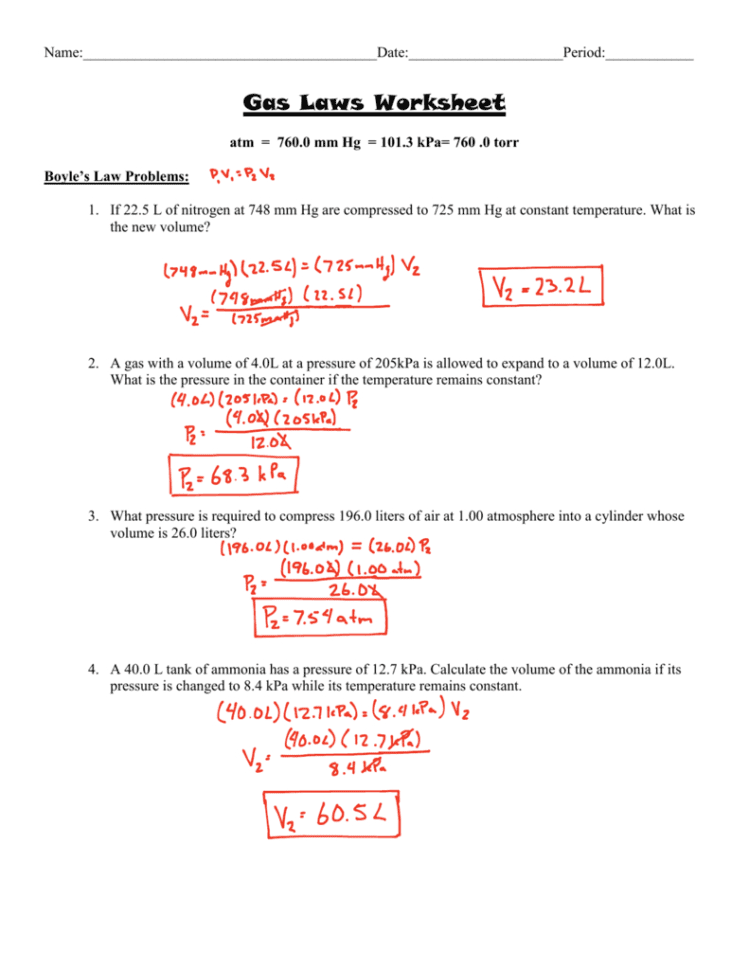
Combined Gas Law Problems Worksheets
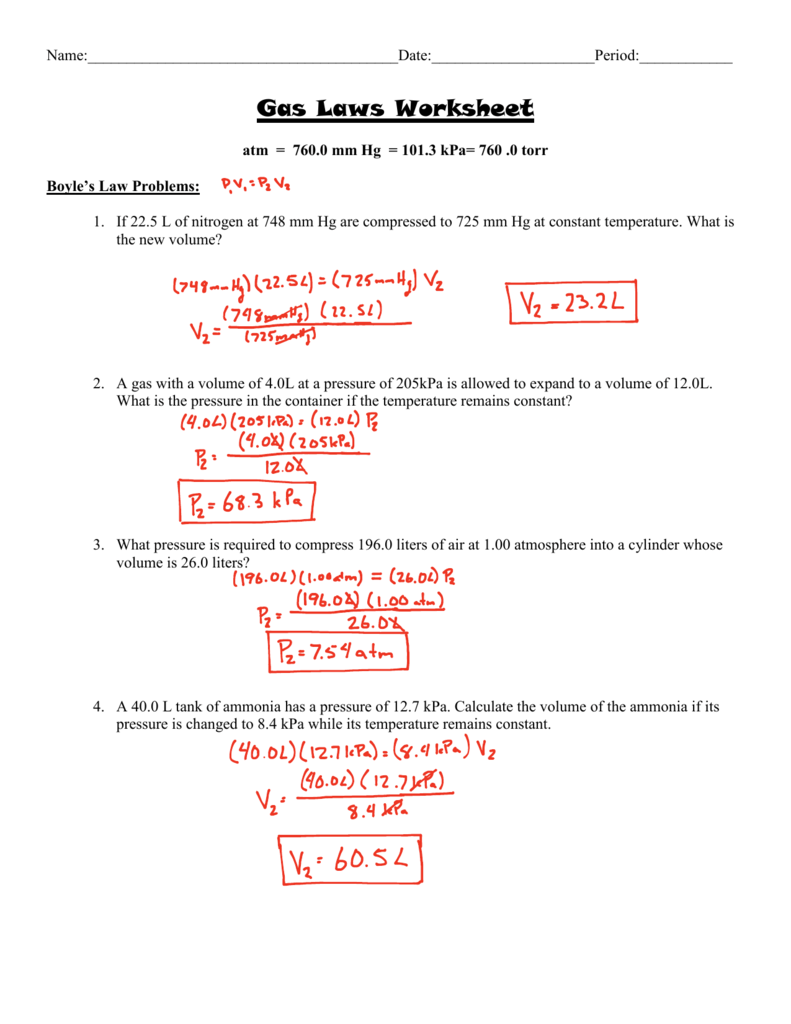
Combined Gas Law Practice Sheet

Chemistry Combined Gas Law Worksheet Answers Ivuyteq

https://www.scribd.com › document
This document provides 9 mixed gas law practice problems involving ideal gas law Dalton s law of partial pressures and Graham s law The problems cover a range of concepts including determining pressure volume moles molar mass and temperature given various gas properties

https://langsdorfscience.weebly.com › uploads ›
MIXED GAS LAWS WORKSHEET 1 How many moles of gas occupy 98 L at a pressure of 2 8 atmospheres and a temperature of 292 K 2 If 5 0 moles of O 2 0and 3 0 moles of N 2 are placed in a 30 0 L tank at a temperature of 25 C what will the
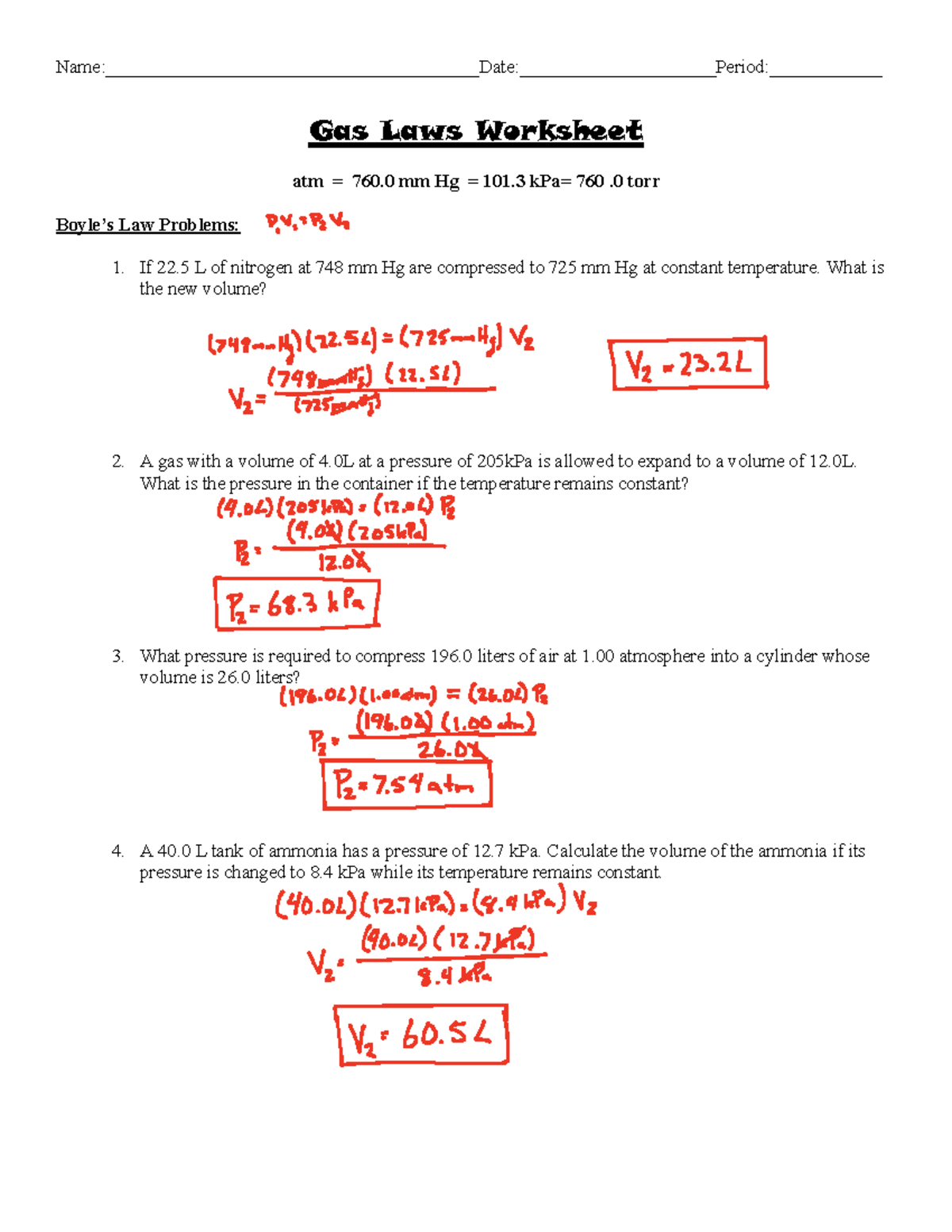
https://www.scribd.com › document › Extra
The document provides 9 practice problems involving the ideal gas law Dalton s law of partial pressures and Graham s law of effusion The problems calculate various gas properties including pressure volume moles grams temperature and molar

https://www.studocu.com › row › document › beirut-arab
Mixed Extra Gas Law Practice Problems Ideal Gas Dalton s Law of Partial Pressures Graham s Law Dry ice is carbon dioxide in the solid state 1 grams of dry ice is placed in a 5 L chamber that is maintained at 35 What is the pressure in the chamber after all
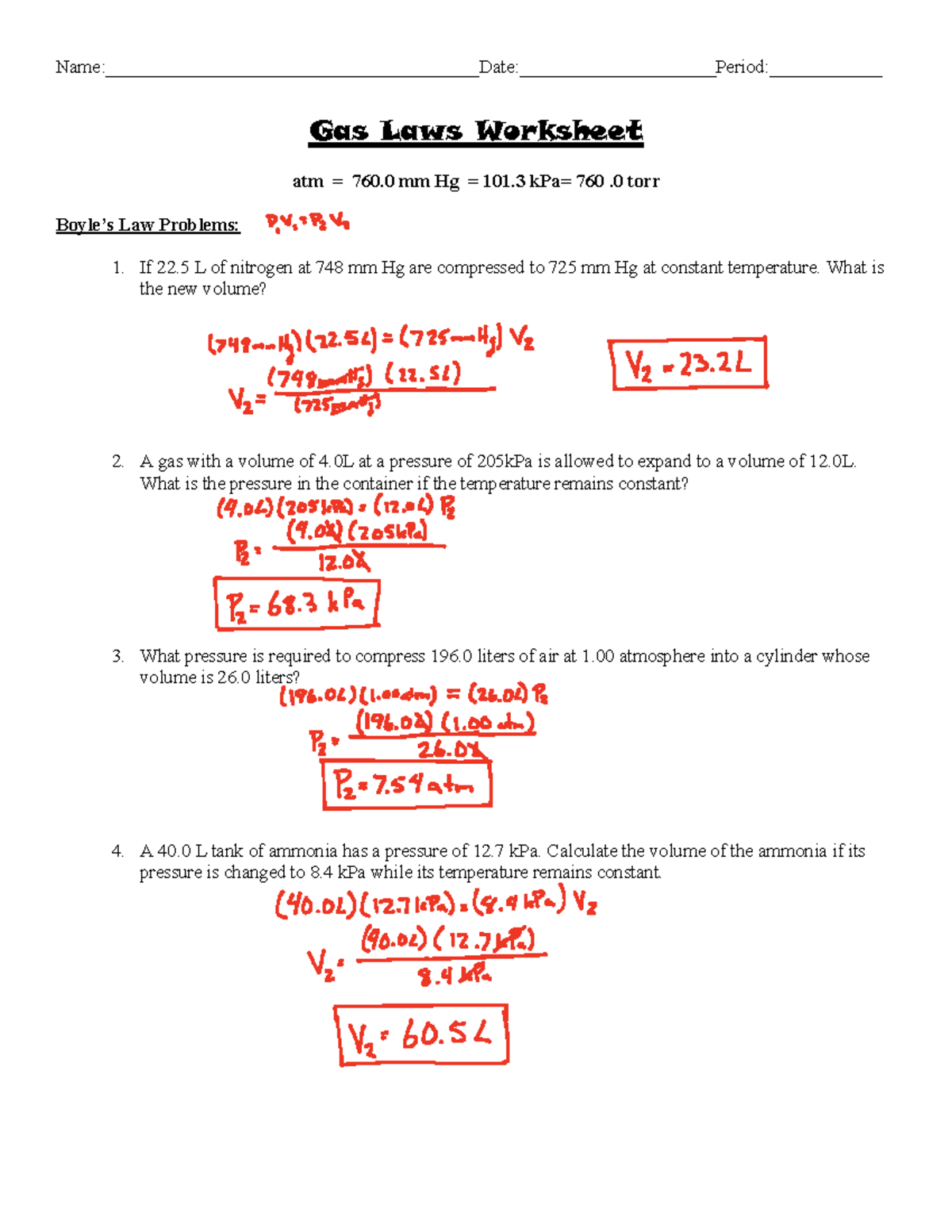
https://www.mcmsnj.net › cms
Mixed Extra Gas Law Practice Problems Ideal Gas Dalton s Law of Partial Pressures Graham s Law 1 Dry ice is carbon dioxide in the solid state 1 28 grams of dry ice is placed in a 5 00 L chamber that is maintained at 35 1oC What is the pressure in the chamber after all of the dry ice has sublimed 2
[desc-11] [desc-12]
[desc-13]