Molarity By Dilution Worksheet Answers Page 69 The density of 3 molal solution of N aOH is 1 110g mL 1 The molarity of the solution is 2 69 M 2 97 M 4 57 M 6 70 M
Molarity can be directly calculated from strength if density is known by weight x 10xd Molarity GMM where d is density and GMM is gram molecular mass Molarity is given by M nsolute V solution here V is the volume of the solution which is temperature dependent
Molarity By Dilution Worksheet Answers Page 69
 Molarity By Dilution Worksheet Answers Page 69
Molarity By Dilution Worksheet Answers Page 69
https://i.pinimg.com/736x/af/66/d4/af66d461f89541622791e6f9a236e3a2.jpg
A commercially available sample of sulphuric acid is 15 sulphuric acid by mass and its density is 1 10g ml Calculate molality and molarity of the solution
Pre-crafted templates offer a time-saving service for producing a varied variety of documents and files. These pre-designed formats and layouts can be utilized for numerous personal and professional projects, including resumes, invites, flyers, newsletters, reports, discussions, and more, streamlining the content creation process.
Molarity By Dilution Worksheet Answers Page 69

7 Molarity Worksheet With Answers Worksheeto

Molarity Practice Worksheet

Key DilutionsWorksheet

4 Ways To Calculate Molarity WikiHow
Molarity Pogil Worksheet Answers

Molarity Definition Calculation Expii

https://www.toppr.com › ask › question › calculatea-molalityb-molarity-an…
Click here to get an answer to your question calculatea molalityb molarity andc mole fraction of ki if the density of 20 mass mass

https://www.toppr.com › ask › question › what-will-be-the-molarity-of-a-s…
a 5 85 g of NaCl is dissolved in 200 mL of water What will be the molarity of this solution b Calculate the molarity of the solution obtained by dissolving 20 6 g NaBr in 500 mL of water
https://www.toppr.com › ask › question
If the concentration of glucose C6H 12O6 in blood is 0 9 g L 1 what will be the molarity of glucose in blood 0 005 M 5 M 0 5 M 50 M
https://www.toppr.com › ask › question › the-relation-between-molarity-…
The molarity and molality of a solution are M and m respectively if the molecular weight of the solute is M calculate the density of the solution in terms of M m and M

https://www.toppr.com › ask › question
Click here to get an answer to your question molarity of 720 gm of pure water is
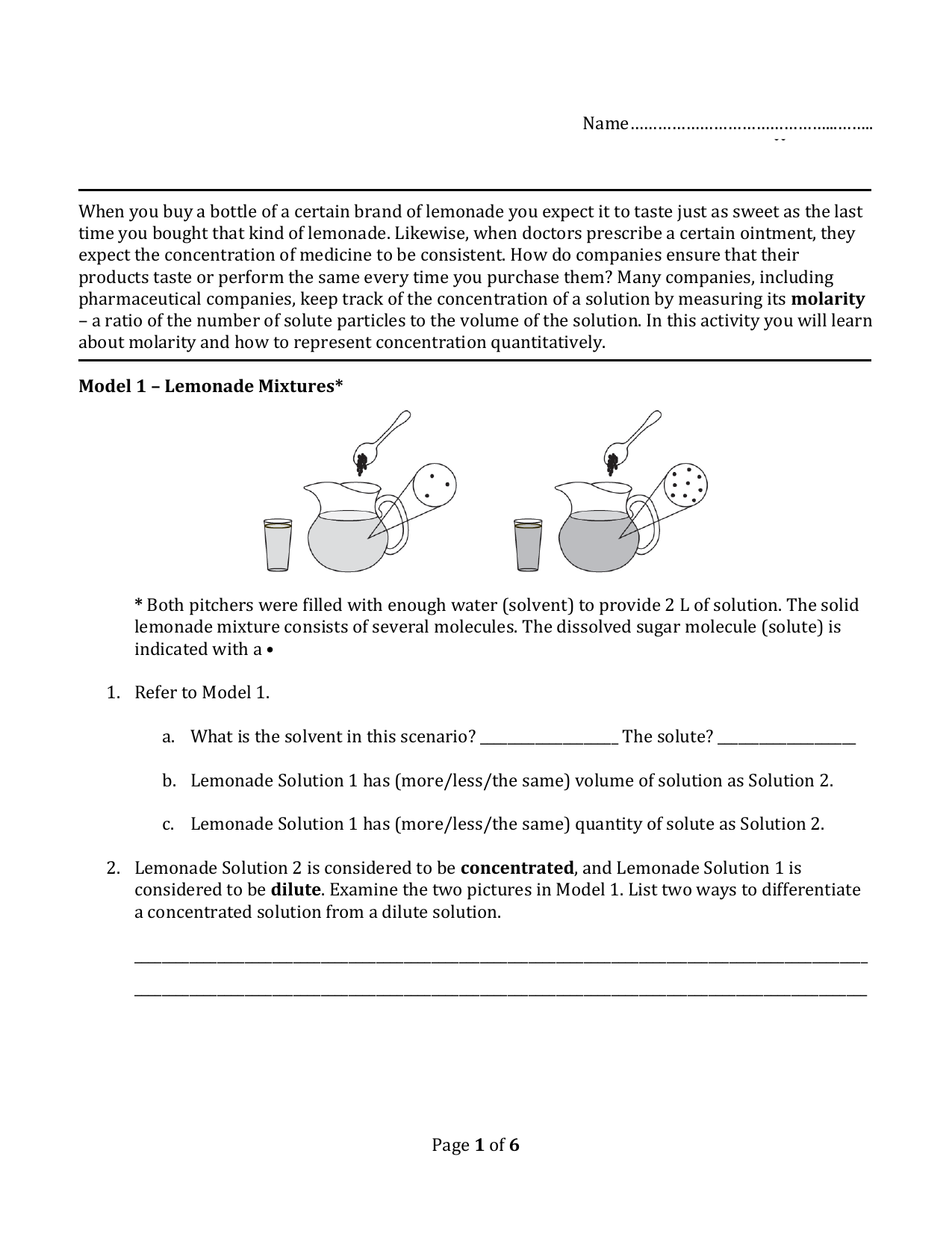
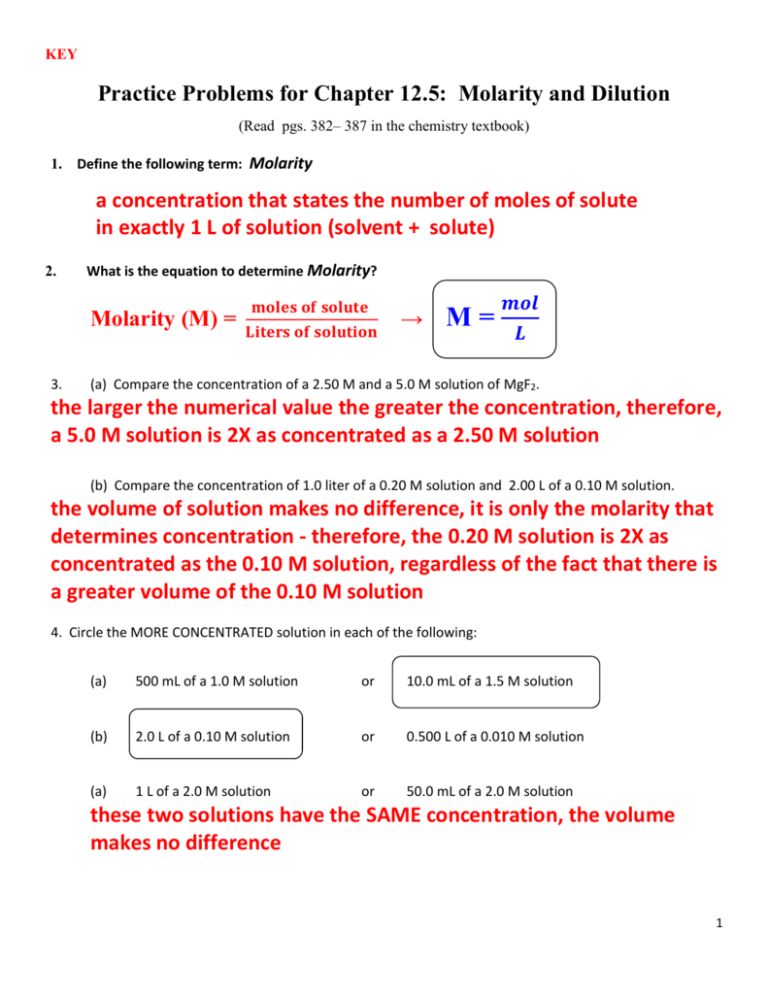
[desc-11] [desc-12]
[desc-13]