Mole Conversion Practice 2 Worksheet Answers Click here to get an answer to your question the number of oxygen atoms present in 50 g of calcium carbonate is
The total number of ions present in 111 g of CaCl2 is one mole two mole four mole three mole Mass of non volatile solute urea needs to be dissolved in 100 g of water in order to decrease the vapour pressure of water by 30 What will be the molality of solution
Mole Conversion Practice 2 Worksheet Answers
 Mole Conversion Practice 2 Worksheet Answers
Mole Conversion Practice 2 Worksheet Answers
https://i.pinimg.com/736x/8d/3f/82/8d3f82a409e95f0b2eeb2fac2bf93f86.jpg
Atomic mass is the concept related to a single atom whereas molecular mass relates to a group of atoms If you are planning to proceed with this concept then it is necessary to gain precise knowledge about molecular mass and mole concept Let us proceed
Templates are pre-designed files or files that can be used for numerous functions. They can conserve time and effort by offering a ready-made format and design for developing various type of content. Templates can be used for individual or expert jobs, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
Mole Conversion Practice 2 Worksheet Answers

Unit 8 Worksheet 1 Mole Relationships Answer Key Kayra Excel
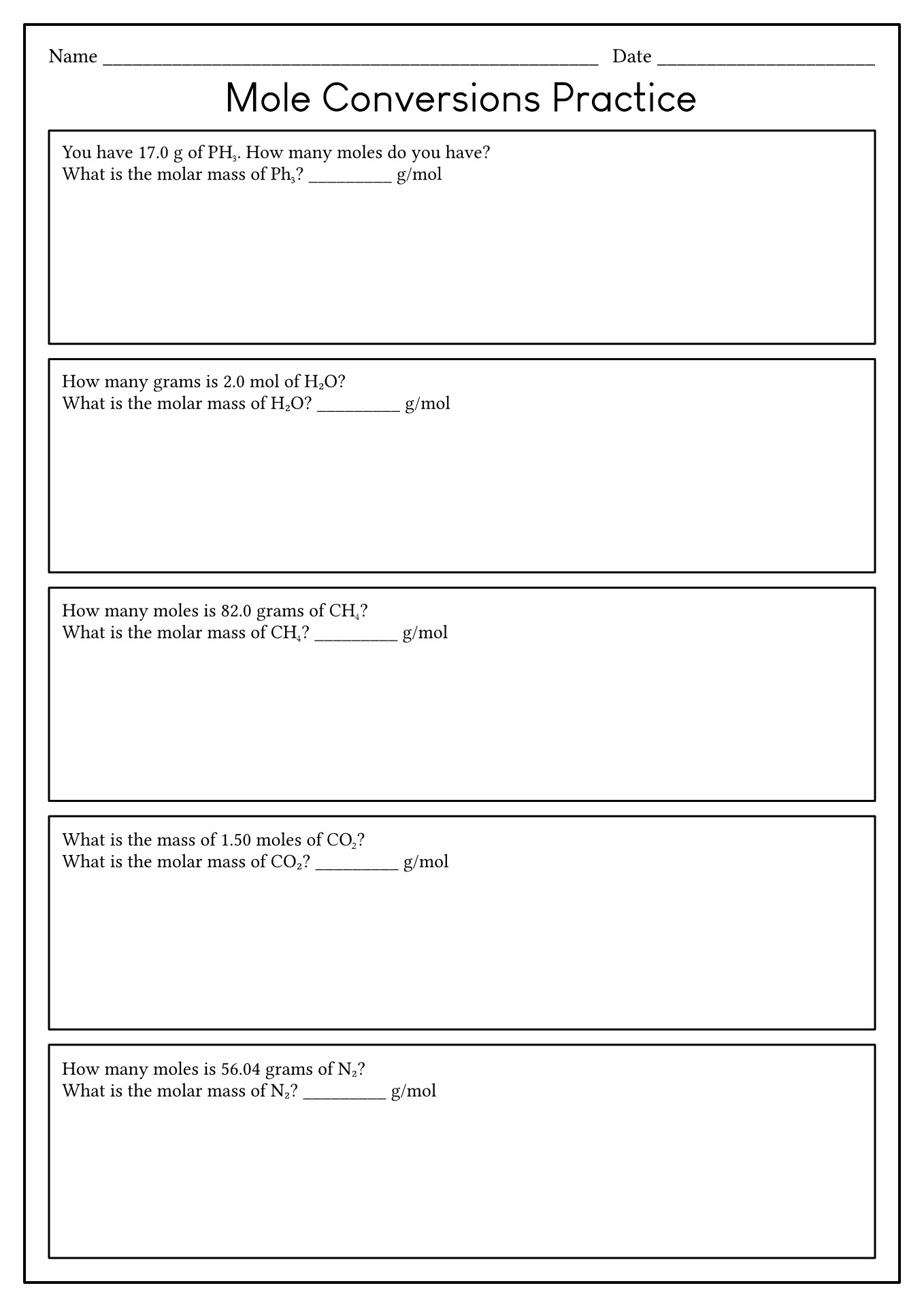
18 Mole Conversion Problems Worksheet Answers Free PDF At Worksheeto

Stoichiometry Mole To Mole Worksheet Answers
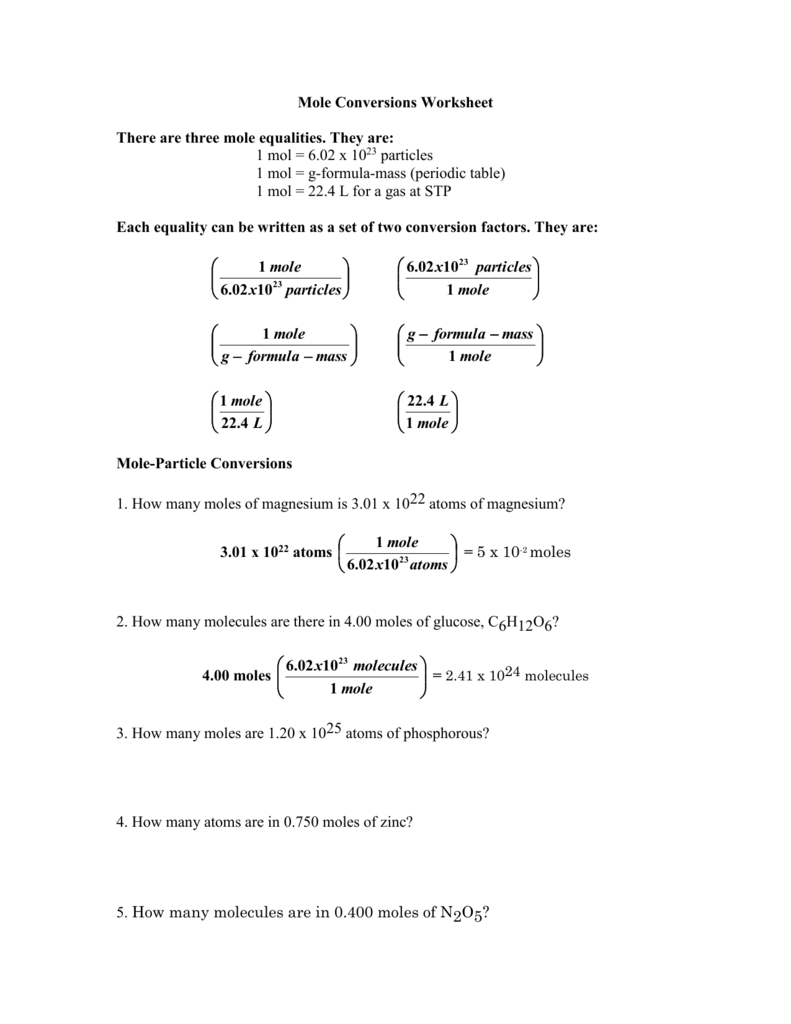
Mole Conversions Worksheet
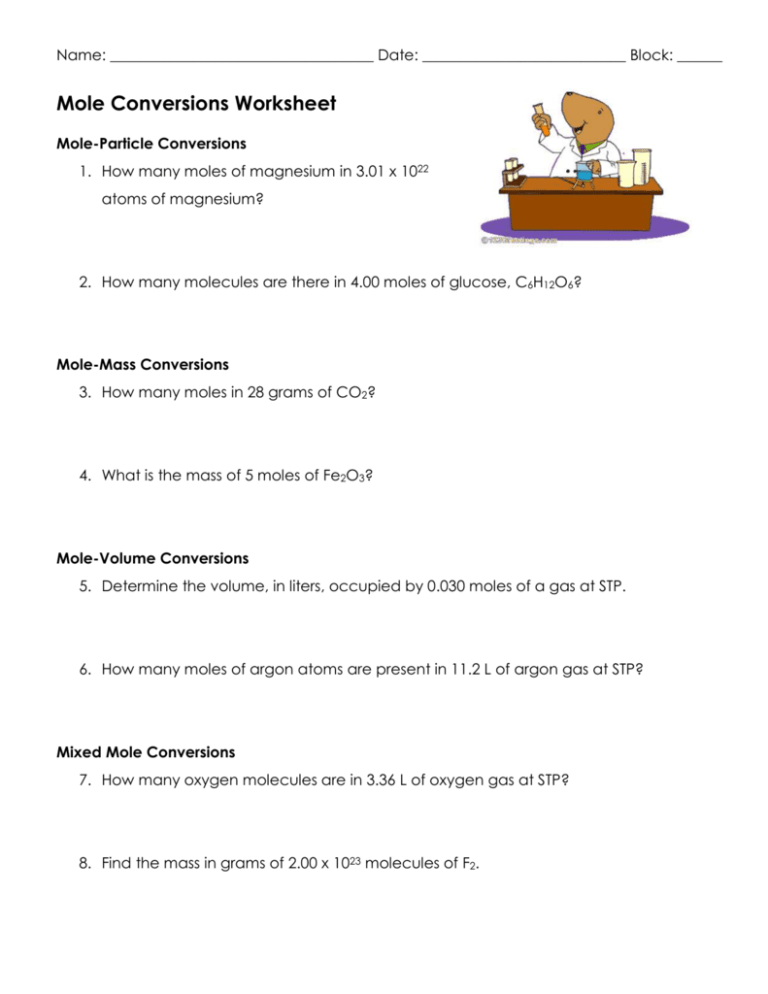
Mole Conversions Worksheet
Moles Molecules And Grams Worksheet Answer Key PDF Worksheet

https://www.toppr.com › ask › question
The vapour pressure of pure liquids 1 and 2 are 550 mm H g and 800 mm H g respectively at 350 K What will be the mole fraction of component 2 in liquid phase when total vapour pressure of the solution is 600 mm H g

https://www.toppr.com › guides › chemistry › some-basic-concepts-of-ch…
Mole and Equivalent Weight If we ask you to count the total number of stars in the sky can you do that No Similarly scientists can not count the exact number of atoms and molecules as they are very tiny and present in a large number Therefore we have the concepts of mole and equivalent weight to make this calculation easy

https://www.toppr.com › ask › question
The vapour pressure of a solvent decreased by 10 mm of Hg when a non volatile solute was added to the solvent The mole fraction of solute in solution is 0 2 what would be the mole fraction of solvent if decrease in vapour pressure is 20 mm of Hg

https://www.toppr.com › ask › question › define-mole-fraction
Define mole fraction A solution of sucrose in water is labelled as 20 w w What would be the mole fraction of each component in the solution

https://www.toppr.com › ask › question
Calculate the mole fraction of glycerin C3H 5 OH 3 in a 100 g solution containing 33 g of glycerin 60 g isopropyl alcohol and rest water Round off to one decimel place
[desc-11] [desc-12]
[desc-13]