Mole Conversion Problems Answer Key Click here to get an answer to your question the number of oxygen atoms present in 50 g of calcium carbonate is
The total number of ions present in 111 g of CaCl2 is one mole two mole four mole three mole Mass of non volatile solute urea needs to be dissolved in 100 g of water in order to decrease the vapour pressure of water by 30 What will be the molality of solution
Mole Conversion Problems Answer Key
 Mole Conversion Problems Answer Key
Mole Conversion Problems Answer Key
http://www.worksheeto.com/postpic/2015/11/mass-to-mole-stoichiometry-worksheet-answer-key_669639.png
Atomic mass is the concept related to a single atom whereas molecular mass relates to a group of atoms If you are planning to proceed with this concept then it is necessary to gain precise knowledge about molecular mass and mole concept Let us proceed
Pre-crafted templates offer a time-saving option for producing a diverse range of files and files. These pre-designed formats and layouts can be utilized for various individual and professional projects, including resumes, invites, flyers, newsletters, reports, discussions, and more, streamlining the content creation procedure.
Mole Conversion Problems Answer Key

11 Moles And Mass Worksheet Answers Worksheeto

Mole Calculation Worksheet Answer Key Worksheets Pinterest

13 Best Images Of Molar Mass Practice Worksheet Answers Mole
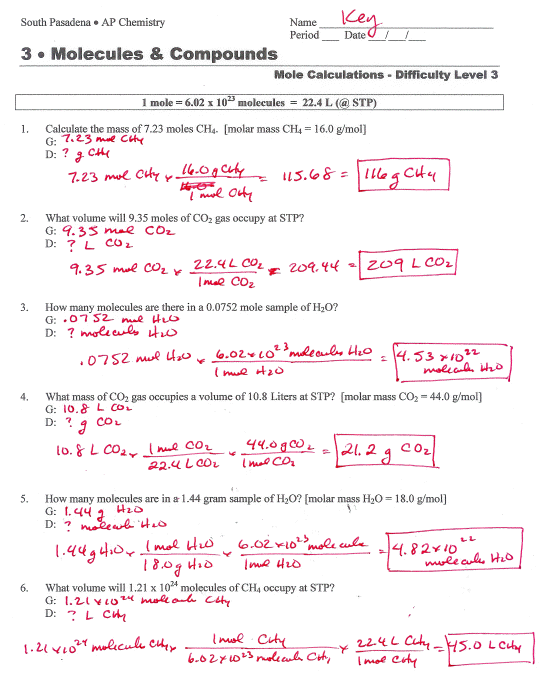
Best Essay Writers Here Solving Molarity Problems Paperillusion web
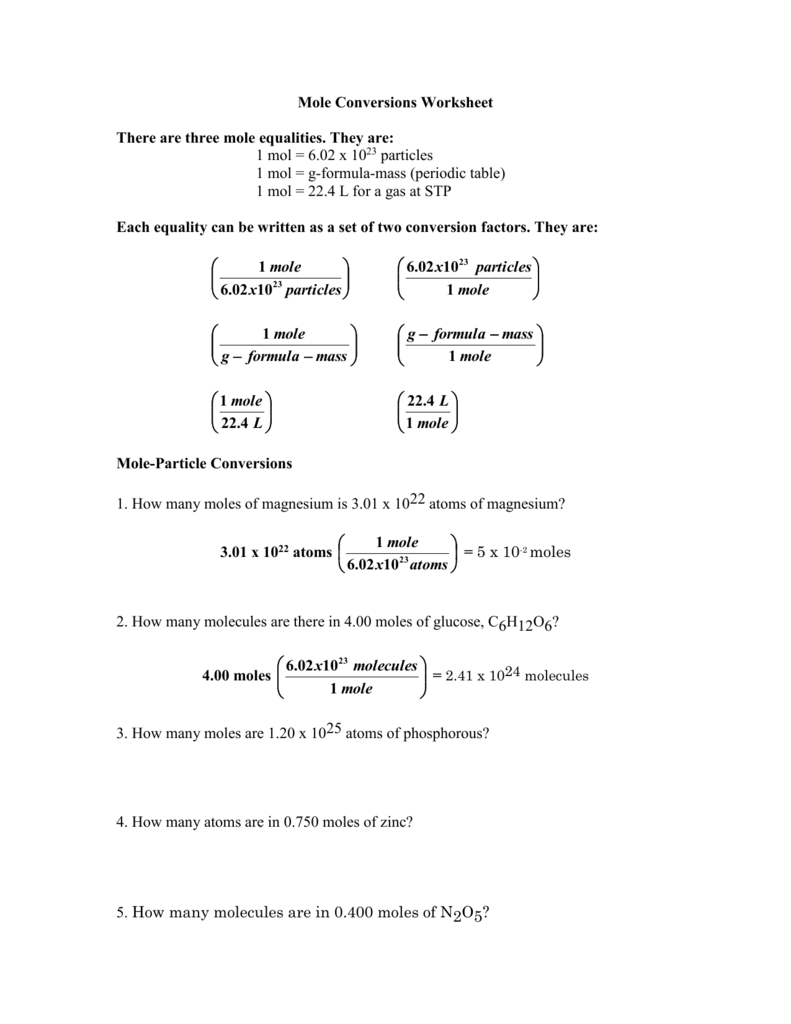
Mole Conversion Worksheet 1
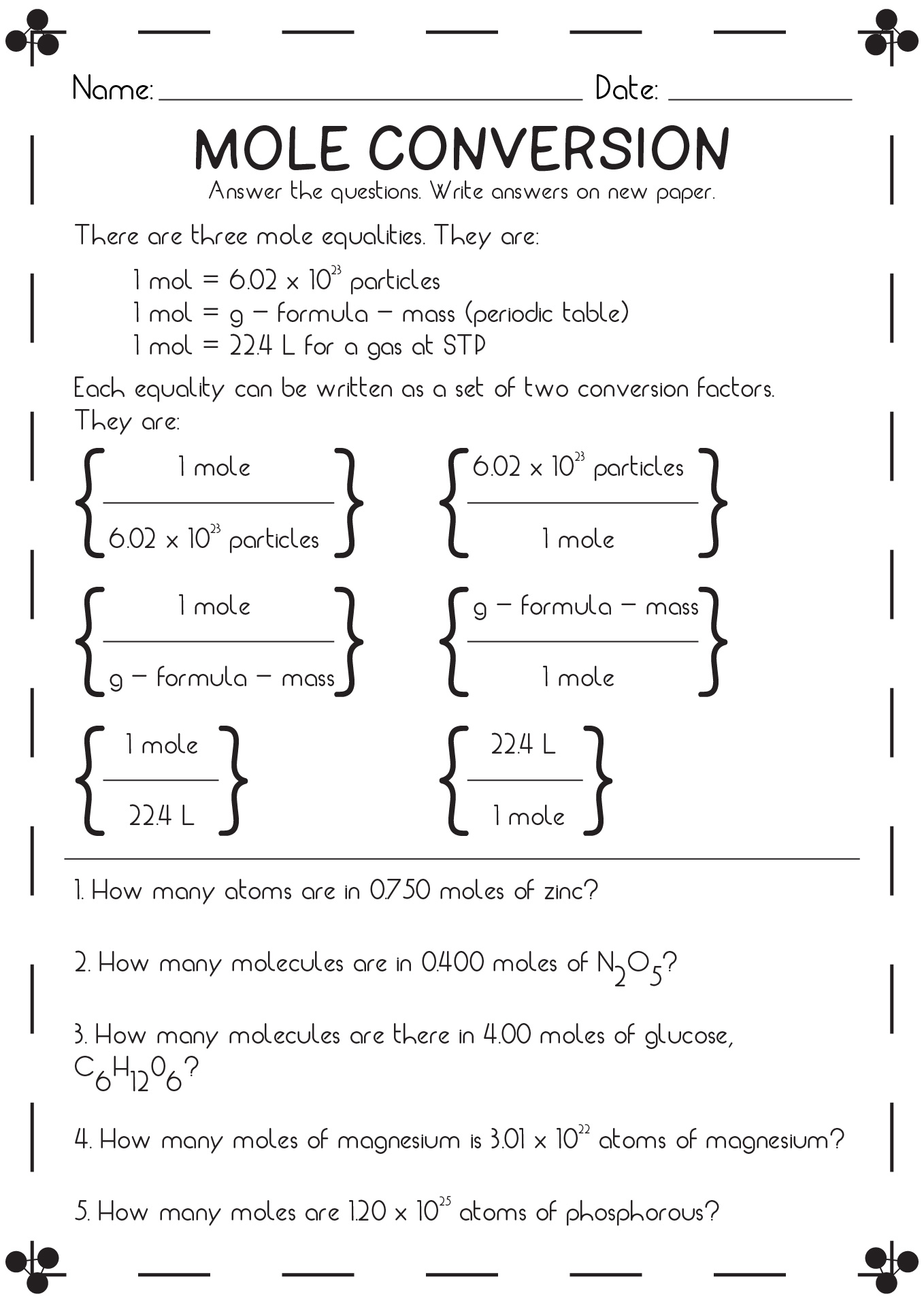
18 Mole Conversion Problems Worksheet Answers Free PDF At Worksheeto

https://www.toppr.com › ask › question
The vapour pressure of pure liquids 1 and 2 are 550 mm H g and 800 mm H g respectively at 350 K What will be the mole fraction of component 2 in liquid phase when total vapour pressure of the solution is 600 mm H g

https://www.toppr.com › guides › chemistry › some-basic-concepts-of-ch…
Mole and Equivalent Weight If we ask you to count the total number of stars in the sky can you do that No Similarly scientists can not count the exact number of atoms and molecules as they are very tiny and present in a large number Therefore we have the concepts of mole and equivalent weight to make this calculation easy

https://www.toppr.com › ask › question
The vapour pressure of a solvent decreased by 10 mm of Hg when a non volatile solute was added to the solvent The mole fraction of solute in solution is 0 2 what would be the mole fraction of solvent if decrease in vapour pressure is 20 mm of Hg

https://www.toppr.com › ask › question › define-mole-fraction
Define mole fraction A solution of sucrose in water is labelled as 20 w w What would be the mole fraction of each component in the solution

https://www.toppr.com › ask › question
Calculate the mole fraction of glycerin C3H 5 OH 3 in a 100 g solution containing 33 g of glycerin 60 g isopropyl alcohol and rest water Round off to one decimel place
[desc-11] [desc-12]
[desc-13]