Mole To Mass Conversion Practice Apr 22 2024 0183 32 The mole symbol mol is the SI unit of amount of substance One mole contains exactly 6 022 140 8 215 1023 elementary entities This number is called the Avogadro number
A mole of a substance has the same mass in grams as one unit atom or molecules has in atomic mass units The mole unit allows us to express amounts of atoms and molecules in visible Feb 13 2025 0183 32 A mole is a fundamental unit in chemistry Learn how to convert between moles and grams and why moles are essential in chemical calculations
Mole To Mass Conversion Practice
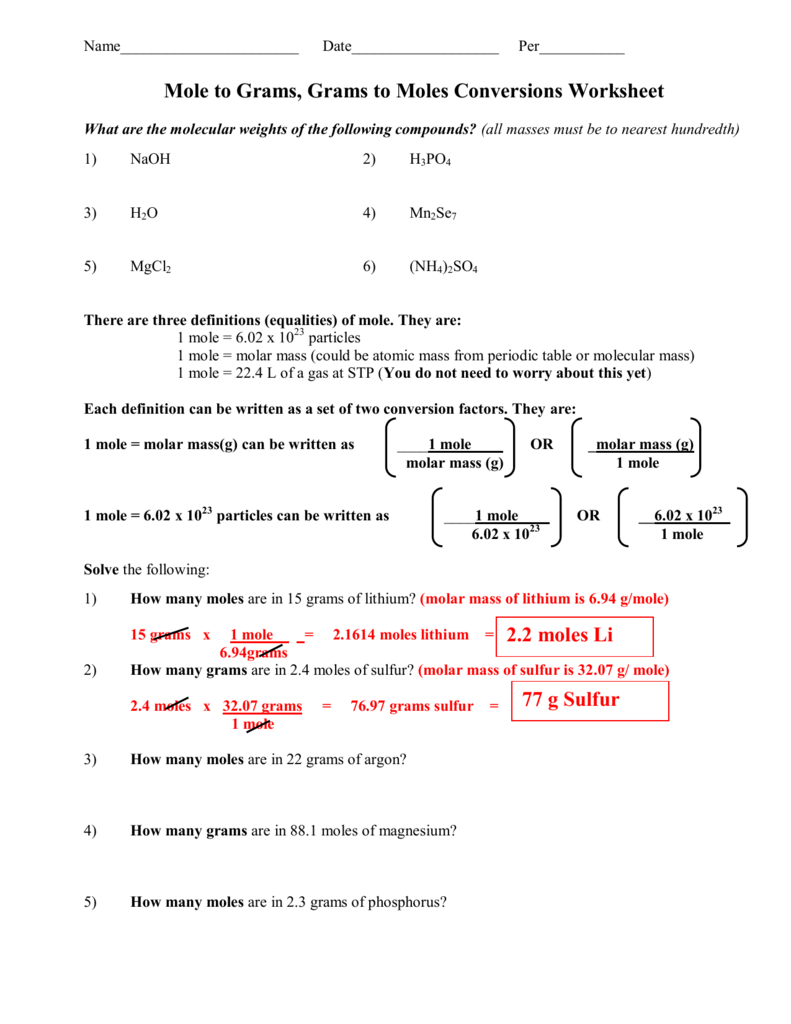 Mole To Mass Conversion Practice
Mole To Mass Conversion Practice
https://s3.studylib.net/store/data/008208304_1-6648c6cb303482b0fdc571fd668e57fc.png
The mass of one mole of atoms molecules ions is called its molar mass M expressed in g mol Numerically the molar mass is equal to the atomic mass of a given atom or a molecule so we
Pre-crafted templates offer a time-saving service for producing a varied series of files and files. These pre-designed formats and layouts can be utilized for different individual and professional jobs, including resumes, invitations, leaflets, newsletters, reports, presentations, and more, simplifying the content creation procedure.
Mole To Mass Conversion Practice

Mole Conversions Worksheet Key

Mass Mole Conversions Worksheet Doc Template PdfFiller
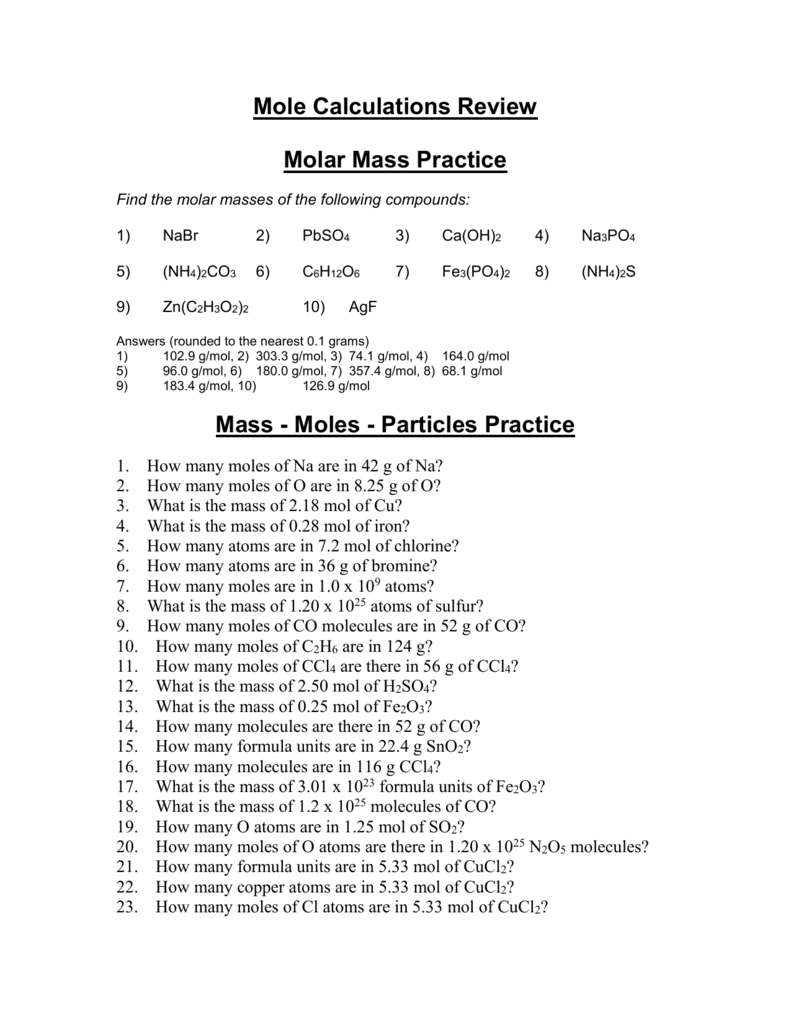
Molar Mass Practice Worksheet

Mole To Liter Conversions Mole To Volume Detailed Examples And

GRAMS TO MOLES CONVERTER CALCULATOR

40 Molar Conversion Worksheet Answers Worksheet Online
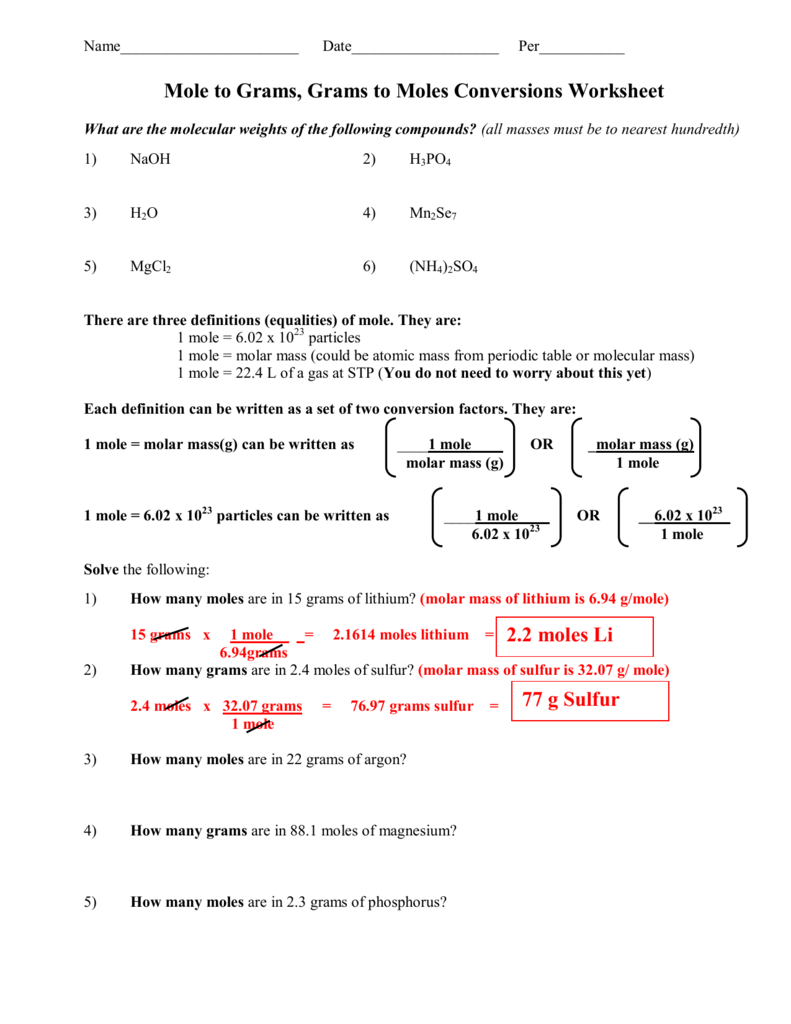
https://www.thoughtco.com
Jul 10 2024 0183 32 Key Takeaways Mole in Chemistry The mole is an SI unit used to measure the amount of any substance The abbreviation for mole is mol One mole is exactly

https://www.bipm.org › en › si-base-units › mole
SI base unit mole mol The mole symbol mol is the SI unit of amount of substance One mole contains exactly 6 022 140 76 x 10 23 elementary entities This number is the fixed numerical

https://themasterchemistry.com › introduction-to-mole-in-chemistry
Dec 1 2023 0183 32 A mole is a unit of measurement that is used to express the amount of substance in a chemical reaction The term mole is defined as the quantity or mass of a substance that

https://scienly.com › what-is-mole
Mar 3 2025 0183 32 A mole mol is a unit of measurement in chemistry that represents a specific number of particles such as atoms molecules or ions A simple definition of mole is as

https://www.sciencenewstoday.org › what-is-the-mole
A mole is the amount of substance that contains the same number of entities atoms molecules ions or electrons as there are atoms in exactly 12 grams of pure carbon 12
[desc-11] [desc-12]
[desc-13]