Mole To Mole Conversion Example One mole of an ideal monoatomic gas undergoes a process described by the equation P V 3 constant The molar heat capacity of this gas during this process is
Calculate the mole fraction of glycerin C3H 5 OH 3 in a 100 g solution containing 33 g of glycerin 60 g isopropyl alcohol and rest water Round off to one decimel place A mixture of ethanol and water contains 54 water by mass Calculate the mole fraction of ethanol in this solution
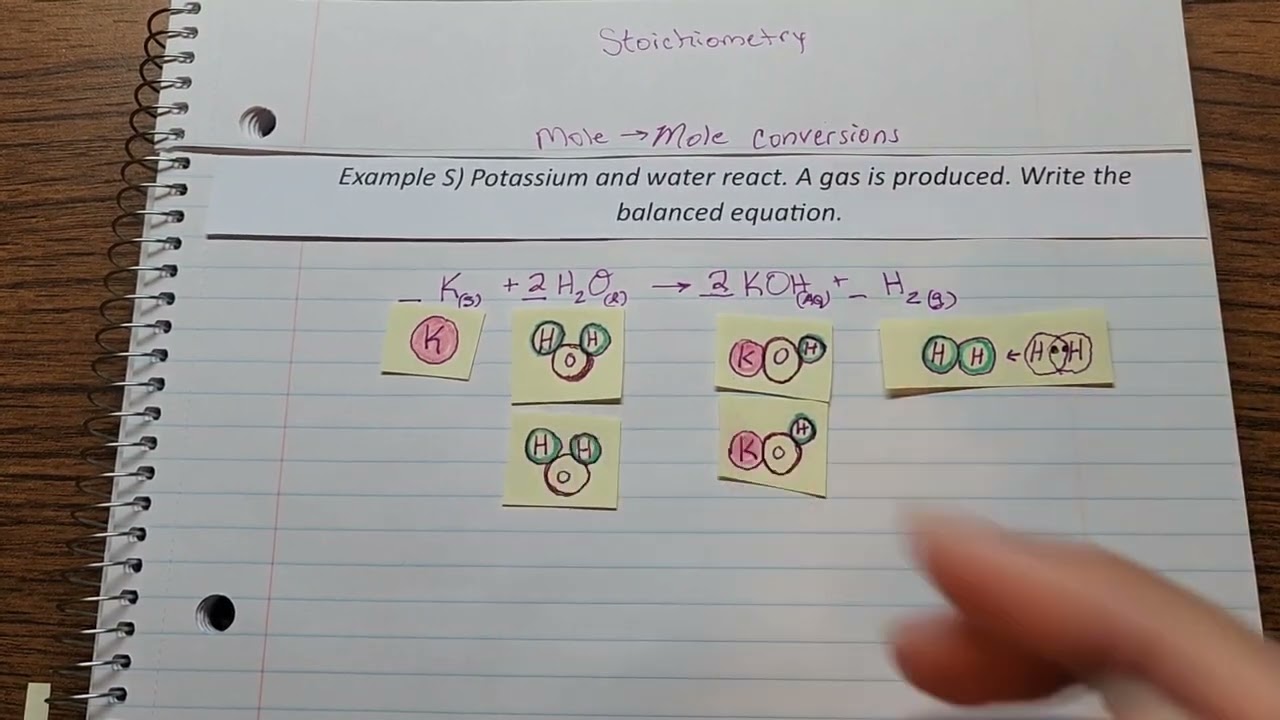
Mole To Mole Conversion Example
 Mole To Mole Conversion Example
Mole To Mole Conversion Example
https://i.ytimg.com/vi/HZw_Mtrrt6g/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGHIgTig7MA8=&rs=AOn4CLDBFflr2bZ10Y5M3wRICcwzlphA2g
The free energy change G when 1 0 mole of water at 100 C and 1 atm pressure is converted into steam at 100 C and 1 atm pressure is 0 cal as the system is at equilibrium
Pre-crafted templates provide a time-saving option for developing a varied range of files and files. These pre-designed formats and designs can be utilized for numerous individual and expert projects, including resumes, invites, leaflets, newsletters, reports, presentations, and more, improving the material development procedure.
Mole To Mole Conversion Example

SimplyChemistry MAP MOLE CONCEPT
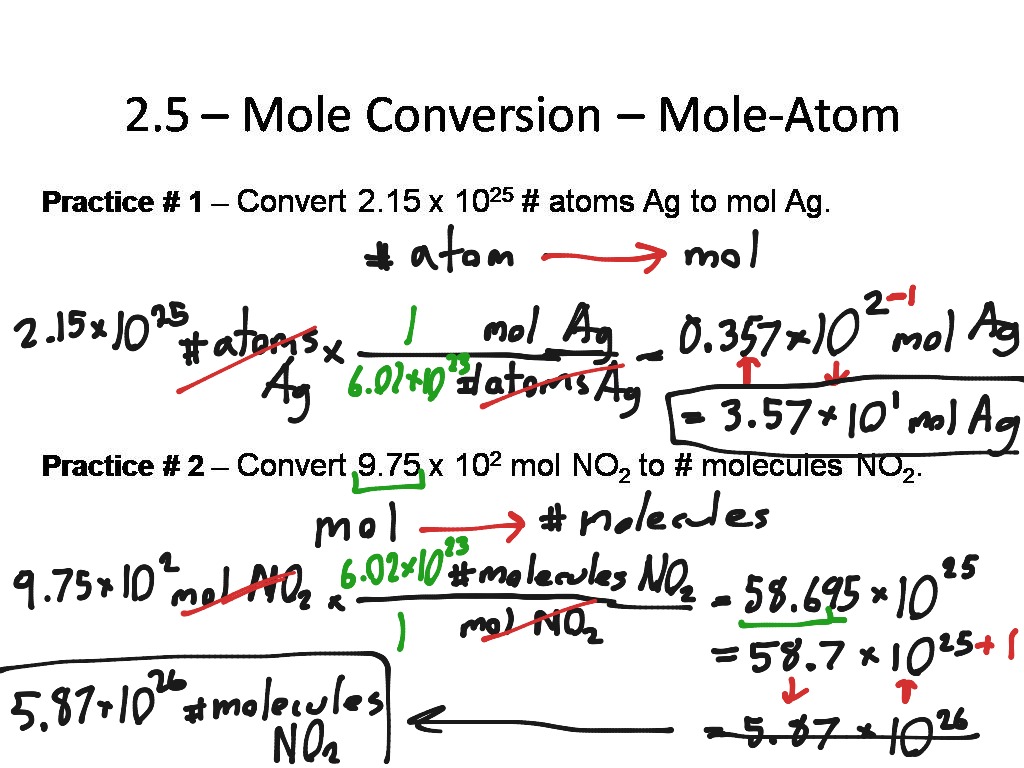
2 5 Mole Conversion Mole Atom 1 Step Science Chemistry ShowMe

Stoichiometry Moles And Mass Science Chemistry Chemical reactions

Percentage Composition Worksheets Answer Key

Chemistry J A K H Mole Conversion

Mole To Mole Stoichiometry Worksheet Key Exercises Chemistry

https://www.toppr.com › ask › question › calculatea-molalityb-molarity-an…
Molality of a solution in aqueous medium is 0 8 Calculate its mole fraction and the percentage by mass of solute if molar mass of solute is 60 View Solution
https://www.toppr.com › ask › question › define-mole-fraction
Define mole fraction A solution of sucrose in water is labelled as 20 w w What would be the mole fraction of each component in the solution

https://www.toppr.com › ask › question › the-combustion-of-one-mole-of …
The combustion of one mole of benzene takes place at 298K and 1 atm after combustion CO2 g and H 2OJ are produced and 3267 0 KJ of heat is liberated Calculate the standard enthalpy of

https://www.toppr.com › ask › question › mole-fraction-of-the-componen…
The mole fraction of component A in vapour phase is x1 and mole fraction of component A in liquid mixture is x2 P 0 A vapour pressure of pure A P 0 B vapour pressure of pure B

https://www.toppr.com › ask › question › what-is-the-symbol-for-si-unit-o…
Symbol for SI Unit of mole is mol One mole is defined as the amount of a substance that contains as many particles or entities as there are atoms in exactly 12 g 0 012 k g of the C 12 isotope
[desc-11] [desc-12]
[desc-13]