Molecular Geometry Practice Worksheet Answers Three dimensional representations inform us about the interactions between different molecules and interactions between different parts of the same molecule In this workshop we will explore one of the models used in chemistry to predict molecular structure the Valence Shell Electron Pair Repulsion model or VSEPR
Species Name Lewis Dot Structure Electronic Arrangement Molecular Geometry BeF 2 linear linear BCl 3 trigonal planar trigonal planar CCl 4 tetrahedral A molecule with the formula AB 2 in which A and B represent different atoms could have one of three different shapes Sketch and name the three different shapes that this molecule might have Give an example of a molecule or ion for each shape Answer
Molecular Geometry Practice Worksheet Answers
 Molecular Geometry Practice Worksheet Answers
Molecular Geometry Practice Worksheet Answers
https://www.semesprit.com/wp-content/uploads/2018/07/molecular-geometry-practice-worksheet-with-answers-as-well-as-ag-science-hypothesis-worksheet-answers-curriculum-of-molecular-geometry-practice-worksheet-with-answers.png
Molecular Geometry Worksheet Summer 2 002 Whelan Page 6 Lewis Structure Classification Electron Pair Geometry EPG Molecular Geometry MG Bond Angle s
Templates are pre-designed files or files that can be utilized for various purposes. They can save effort and time by supplying a ready-made format and layout for creating different kinds of content. Templates can be utilized for personal or expert tasks, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
Molecular Geometry Practice Worksheet Answers
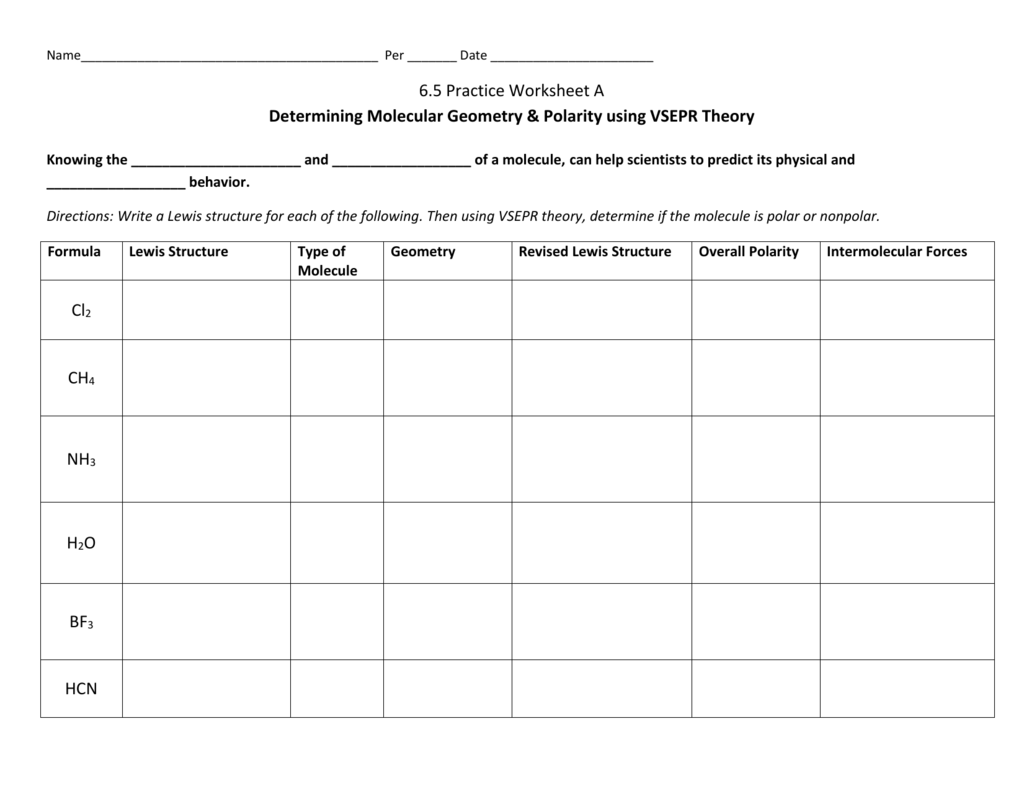
6 5 Practice Worksheet A Molecular Geometry Polarity

VSEPR Theory And Molecular Geometry Vsepr Theory Molecular Geometry

Molecular Geometry Practice Worksheet With Answers Kidsworksheetfun
Vsepr Worksheet With Answers
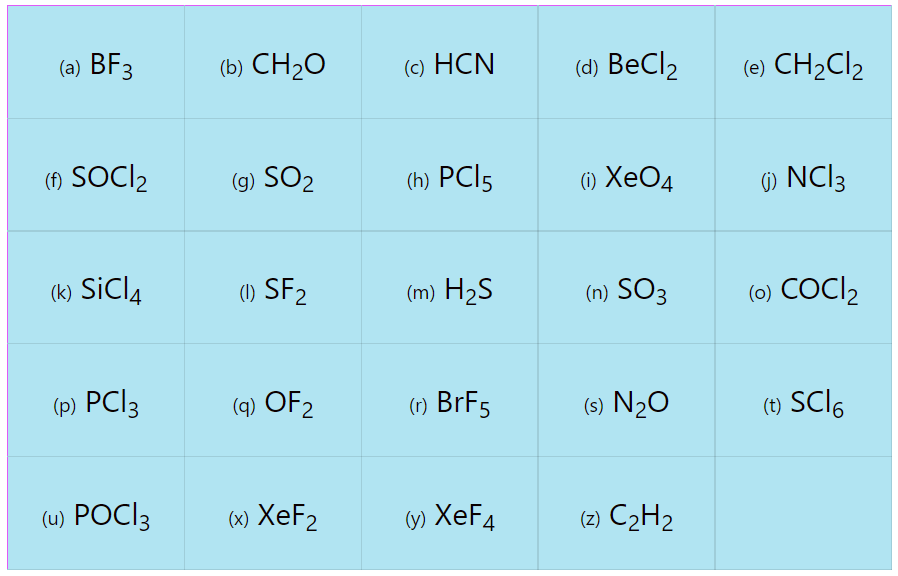
Vsepr Worksheet With Answers Worksheets For Kindergarten
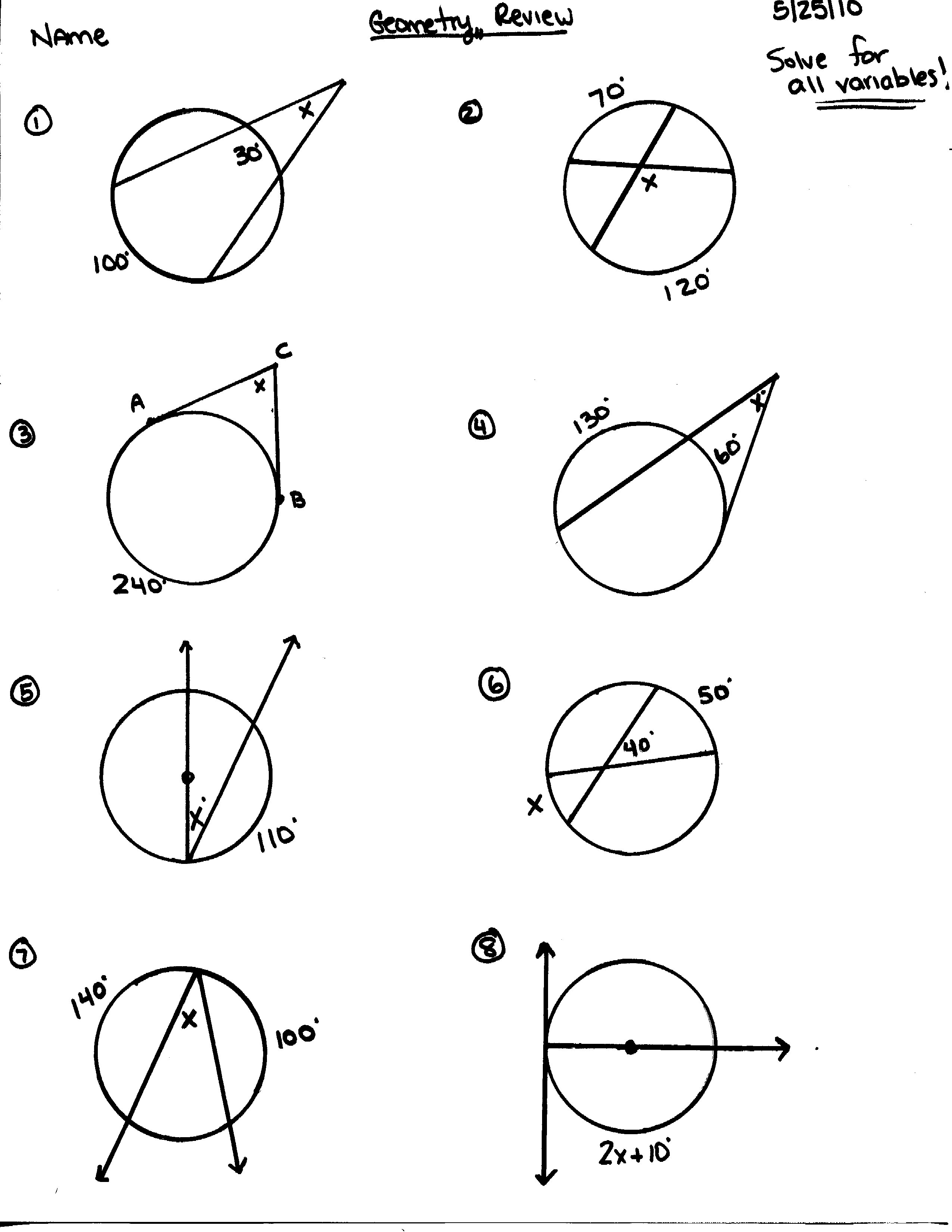
12 Best Images Of Circle Arcs And Angles Worksheets Geometry Circle

http://butane.chem.uiuc.edu//Worksheet13_VSEPR_Key.pdf
1 Draw the Lewis structure for water H2O H O H a How many quot groups quot atoms and lone pairs surround the central oxygen 4 b What is the geometry of this molecule look at atoms and lone pairs Draw this VSEPR structure next to the Lewis structure tetrahedron c What is the shape of this molecule look only at the atoms bent

https://www.teachengineering.org/content/fiu
Lewis Dot Structures and Molecule Geometries Worksheet Answer Key How to Draw a Lewis Dot Structure Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion You can quickly refer to the periodic table for the group A number for this information

https://www.chem.purdue.edu/vsepr/practice.html
Give the name of the electronic arrangement and the name for the molecular geometry for each of the species in question 1 Draw the best Lewis Dot Structures for each of the following species a Give the name of the electronic arrangement and the name for the molecular geometry for each of the species in question 3
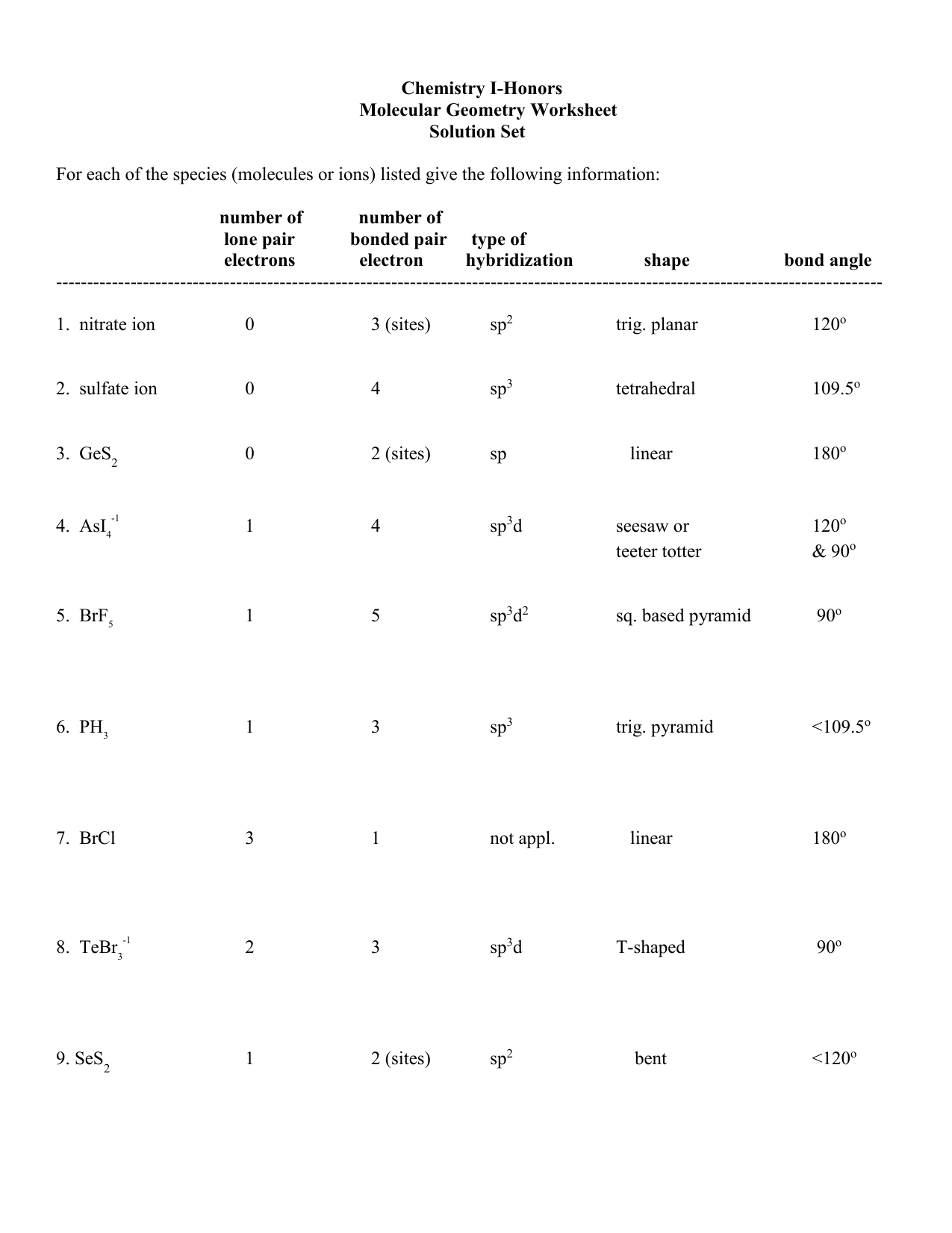
https://www.khanacademy.org/science/ap-chemistry
Choose 1 answer Compounds 1 and 2 are both polar A Compounds 1 and 2 are both polar Compounds 1 and 2 are both nonpolar B Compounds 1 and 2 are both nonpolar Compound 1 is polar whereas compound 2 is nonpolar C Compound 1 is polar whereas compound 2 is nonpolar Compound 1 is nonpolar whereas compound 2 is polar D

https://www.chem.purdue.edu/vsepr
All parts of the assignment Molecular Geometry Lab Parts I II a II b and III are to be answered in your lab notebook You should follow a specific format for entering your answers in your notebook You can access any part of
Molecular Geometry VSEPR Theory Practice Problems In these practice problems we will work on determining the electron and molecular geometry of molecules and ions using the VSEPR theory Practice 1 Determine the electron geometry and molecular geometry of the following molecules using the VSEPR model a answer The arrangement of atoms in several biologically important molecules is given here Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs Do not add any more atoms a the amino acid serine b urea c pyruvic acid d uracil e carbonic acid Answer a Answer b Answer c Answer d Answer e
BONUS Mathematical Operations and Functions 0 Multiplication and Division Operations 0 Addition and Subtraction Operations 0 Power and Root Functions 0 Power and Root Functions 0 The Quadratic Formula 0 Learn Molecular Geometry Simplified with free step by step video explanations and practice problems by experienced tutors