Multiply 3 Digit Numbers Trick Download Free PDF Thermodynamics of NaCl in steam Roberto Pabalan An Empirical Model of the Gibbs Free Energy for Solutions of NaCl and CaCl2 of Arbitrary Concentration at
Lecture Notes on Thermodynamics 201 ric Brunet1 Thierry Hocquet2 Xavier Leyronas3 February13 2019 Atheoryisthemoreimpressivethegreaterthesimplicityofitspremisesis CHM2 11 12 LAB12 Q4 FD Free download as PDF File pdf Text File txt or read online for free This laboratory worksheet focuses on observing entropy changes in chemical reactions
Multiply 3 Digit Numbers Trick
 Multiply 3 Digit Numbers Trick
Multiply 3 Digit Numbers Trick
https://i.pinimg.com/originals/89/e1/0b/89e10bd88341892b526ca2261c35d3d2.jpg
[desc-9]
Templates are pre-designed documents or files that can be used for numerous purposes. They can save time and effort by offering a ready-made format and design for creating various sort of material. Templates can be used for personal or expert jobs, such as resumes, invitations, leaflets, newsletters, reports, discussions, and more.
Multiply 3 Digit Numbers Trick
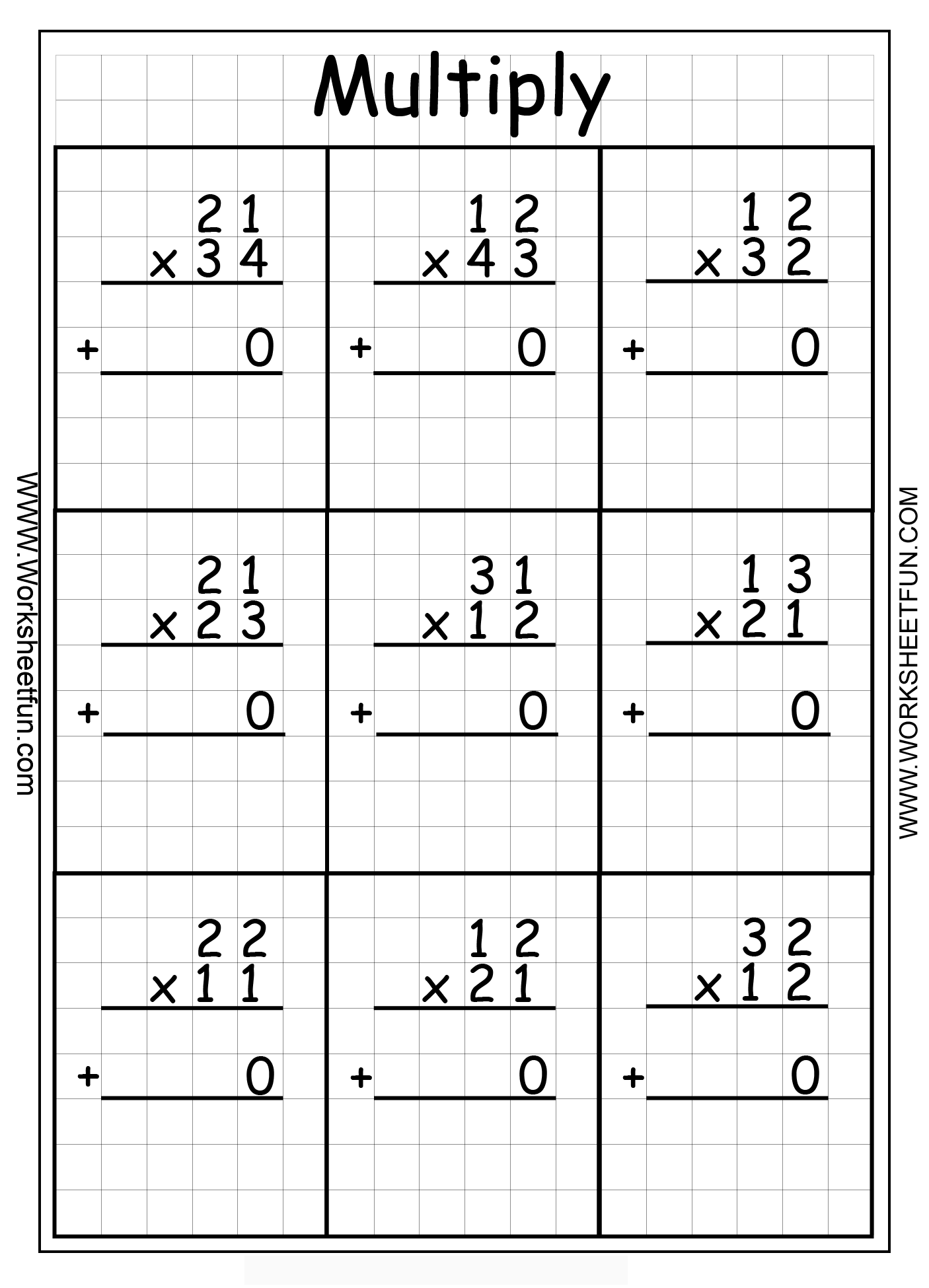
Multiply Two Digit By One Digit Worksheets

2 Digits Fast Multiplication Trick Easy And Fast Way To Learn

How To Multiply Triple Digits
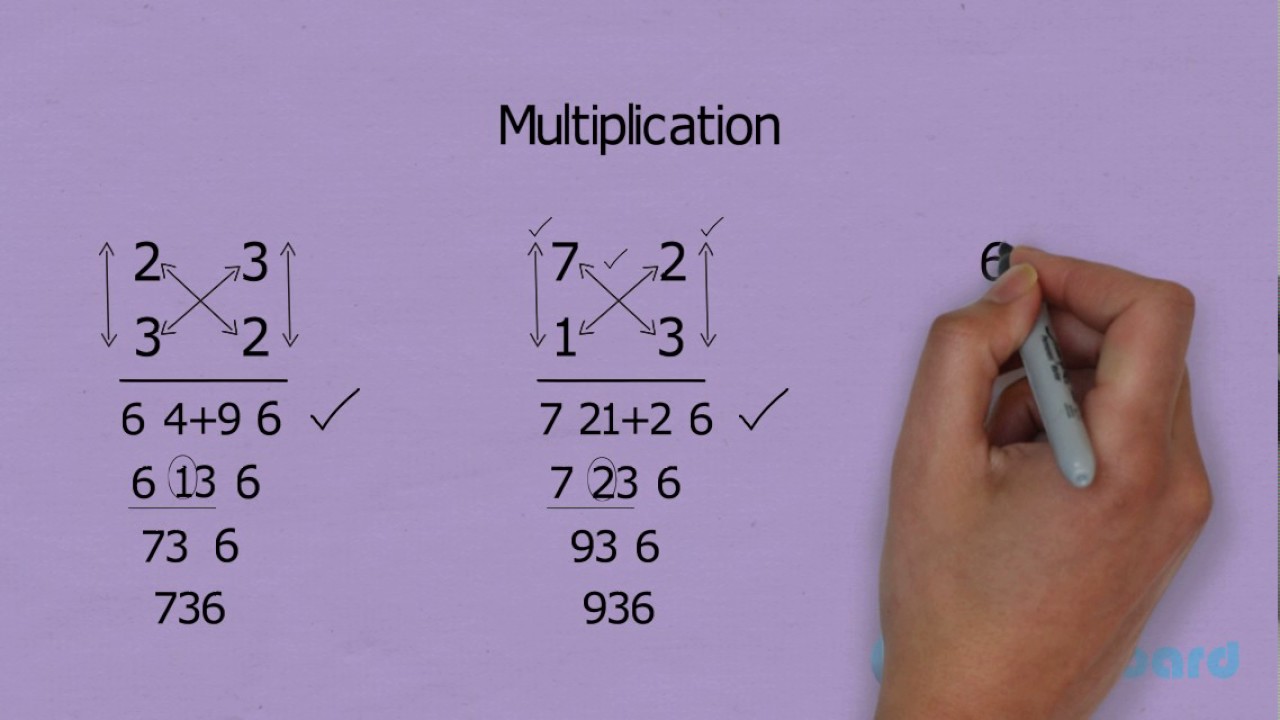
Multiply By 3 Digit Numbers
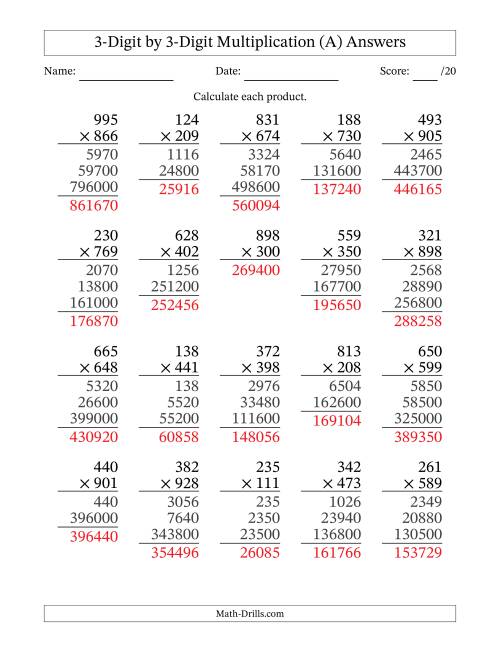
3 Digit Number Multiplication

Three Digit By 2 Digit Multiplication

https://www.drjez.com › uco › ChemTools › Standard Ther…
32 Formula State of Matter Enthalpy kJ mol Entropy J mol K Gibbs Free Energy kJ mol Al NO 3 3 6H 2O s 2850 47552 467 7712 2203 88016 Al NO 3 3 9H 2O s 3757 06464 569 024

https://link.springer.com › content › pdf
Thermodynamics of Electrolytes 13 Ionic Strength Dependence of Higher Order Terms Equations for CaCl2 and MgCl2 Kenneth S Pitzer 1 4 Peiming Wang 1 Joseph A Rard 2 and

https://arxiv.org › pdf
The former is typically employed to explore the solvation structure and thermodynamics e g solvation energy redox potential etc 13 14 This approach heavily relies on ion charge
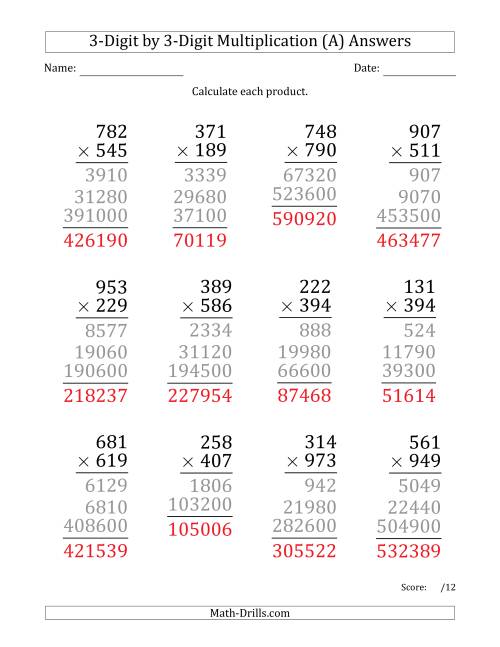
https://drclays-alevelchemistry.com › › Thermodynaimc…
Thermodynamics Past Pape Questions ii Use the cycle in part i and the data in the table to calculate a value in kJ mol 1 for the bond enthalpy of the fluorine fluorine bond Enthalpy

https://scholar.harvard.edu › files › schwartz › files
2Heat Saywehavetwosystemswithdi erenttemperaturesT 1andT 2 energiesE 1andE 2andnumbers ofparticlesN 1andN 2 Thenumberofmicrostatesofeachis 1 N 1 E 1 and 2 N 2 E 2 andtheir
[desc-11] [desc-12]
[desc-13]