Percent Composition Empirical And Molecular Formulas Worksheet Answers Worksheet 7 3 Name Percent Composition Empirical Formulas Period Glencoe Chemistry pp 328 337 Show your work to receive credit Circle your final answer
An unknown compound was found to have a percent composition as follows 47 0 potassium 14 5 carbon and 38 5 oxygen If the true molar mass of the Chemistry Percentage Composition and Empirical Molecular Formula Solve the Find the molecular formula for ethane Answers 1 Na2SO3 6 H3PO4 2 Fe2S3
Percent Composition Empirical And Molecular Formulas Worksheet Answers
 Percent Composition Empirical And Molecular Formulas Worksheet Answers
Percent Composition Empirical And Molecular Formulas Worksheet Answers
https://ecdn.teacherspayteachers.com/thumbitem/Percent-Composition-Empirical-Formulas-and-Molecular-Formulas-Worksheet-1846559-1516541177/original-1846559-1.jpg
Percent Composition and Molecular Formula Worksheet 1 What s the empirical formula of a molecule containing 65 5 carbon 5 5 hydrogen and 29 0 oxygen
Templates are pre-designed documents or files that can be utilized for numerous purposes. They can save time and effort by offering a ready-made format and design for producing various sort of content. Templates can be utilized for personal or professional jobs, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
Percent Composition Empirical And Molecular Formulas Worksheet Answers

Answer Key Empirical and Molec Formula WS.pdf | Exercises Chemistry | Docsity

Percent Composition and Molecular Formula Worksheet Key | PDF

Percent Composition & Empirical & Molecular Formulas Worksheets (Set of 2)

Percentage Composition and Empirical & Molecular Formula Worksheet for 10th - 12th Grade | Lesson Planet

ColorByNumberQuestionsAndKEY.pdf - Percent Composition Empirical & Molecular | Course Hero

Solved Empirical and Molecular Formula Worksheet Show ALL | Chegg.com

https://jseguin.weebly.com/uploads/3/1/9/7/3197205/__comp_empirical_and_molecular_worksheet_-answer_key.doc
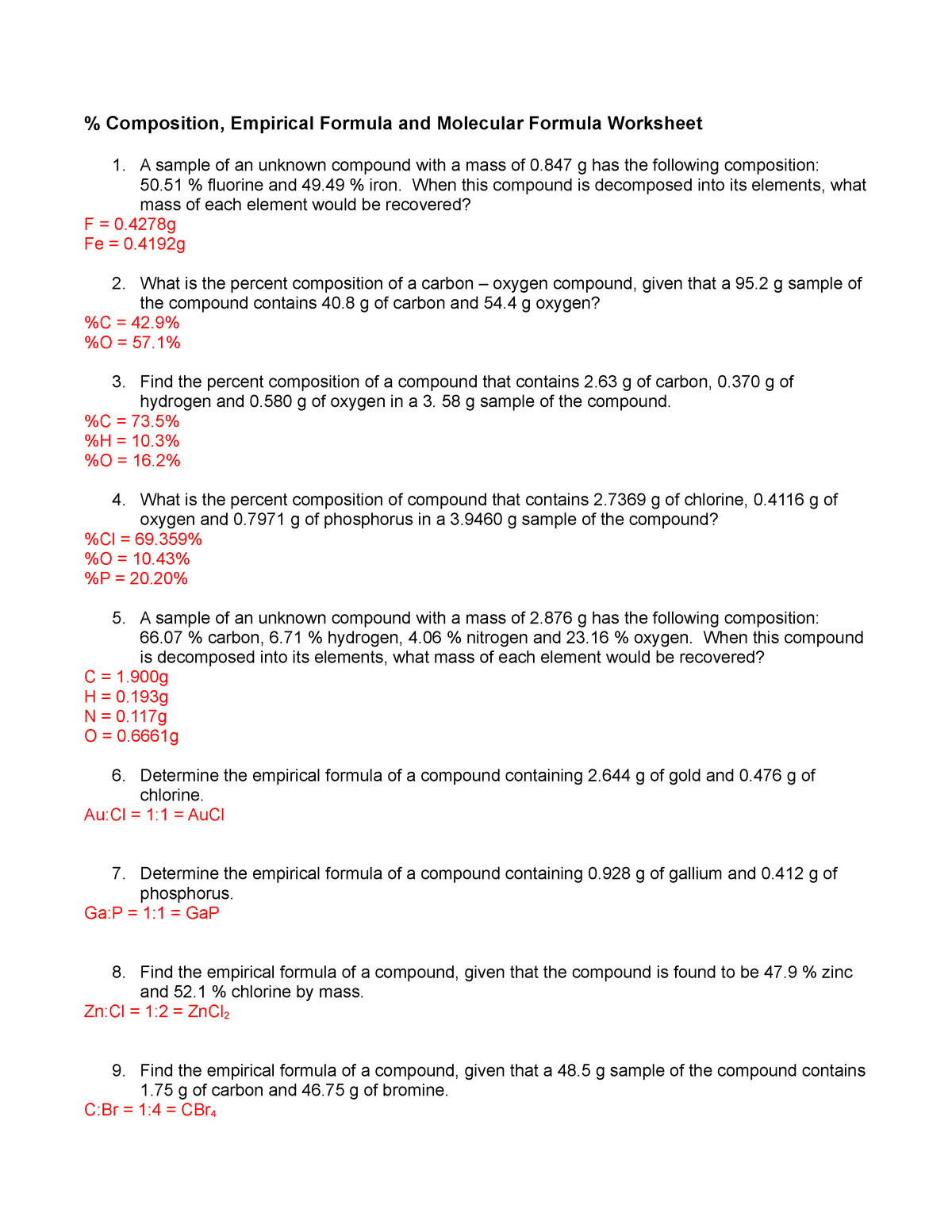
Composition Empirical Formula and Molecular Formula Worksheet A sample of an unknown compound with a mass of 0 847 g has the following composition 50 51

https://www.ahschools.us/cms/lib/MN01909485/Centricity/Domain/4810/The%20Mole%20and%20Stoich/Calculationg%20Empirical%20and%20Molecular%20Formulas%20WS%20Key.pdf
Calculate the empirical formula of a compound that is 85 6 C and 14 4 H by mass B CH C C3H5 D C H4 E C H 282 11 01

https://chem.libretexts.org/Courses/Los_Angeles_Trade_Technical_College/DMA_Chem_51_Su_19/2%3A_Beginning_Chemistry_(Ball)/05%3A_Stoichiometry_and_the_Mole/5.4_Percent_Composition%2C_Empirical_and_Molecular_Formulas
What is this compound s percent composition Answer 12 1 C 16 1 O 71 8 Cl Determining Percent Composition from Formula Mass

https://www.chemistrylearner.com/worksheets/percent-composition-and-empirical-formula-worksheets
These worksheets are designed to test students knowledge of percent composition and empirical formulas of compounds They are tested on various problems
https://www.kenwoodacademy.org/ourpages/auto/2012/3/19/49893888/Percent%20Composition%20and%20Empirical%20Formula%20Calculation%20Wkst.doc
Answer the following questions The percentage composition of acetic acid is found to be 39 9 C 6 7 H and 53 4 O Determine the empirical
What is the empirical and molecular formula for sucralose The percent composition is 36 25 C 4 82 H 26 75 Cl and 32 19 O The molar mass is 397 63 g mole Step 3 Use this number and multiply the subscripts in the empirical formula to arrive at the molecular formula 6 CH2O C6H12O6 Correct answer C6H12O6
This chemistry video tutorial explains how to find the empirical formula given the mass in grams