Percent Composition Empirical And Molecular Formulas Worksheet Color By Number WEB Find the percent composition of a compound that contains 2 63 g of carbon 0 370 g of hydrogen and 0 580 g of oxygen in a 3 58 g sample of the compound 73 5 C 10 4 H 16 2 O
WEB Percent Composition Empirical amp Molecular Formulas Color By Number Add art and creativity to your review lesson Students work ten problems choose the correct answer and color their masterpieces according to the color beside the correct answer WEB Molecular Formula Worksheet Write the molecular formulas of the following compounds 1 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole 2 A compound with an empirical formula of
Percent Composition Empirical And Molecular Formulas Worksheet Color By Number
 Percent Composition Empirical And Molecular Formulas Worksheet Color By Number
Percent Composition Empirical And Molecular Formulas Worksheet Color By Number
https://d20khd7ddkh5ls.cloudfront.net/empirical_formula_examples.jpeg
WEB Percent Composition Empirical amp Molecular Formulas Color By Number Add art and creativity to your review lesson Students work ten problems choose the correct answer and color their masterpieces according to the color beside the correct answer
Pre-crafted templates offer a time-saving option for developing a varied range of files and files. These pre-designed formats and designs can be used for numerous individual and expert jobs, consisting of resumes, invitations, flyers, newsletters, reports, presentations, and more, improving the material creation process.
Percent Composition Empirical And Molecular Formulas Worksheet Color By Number

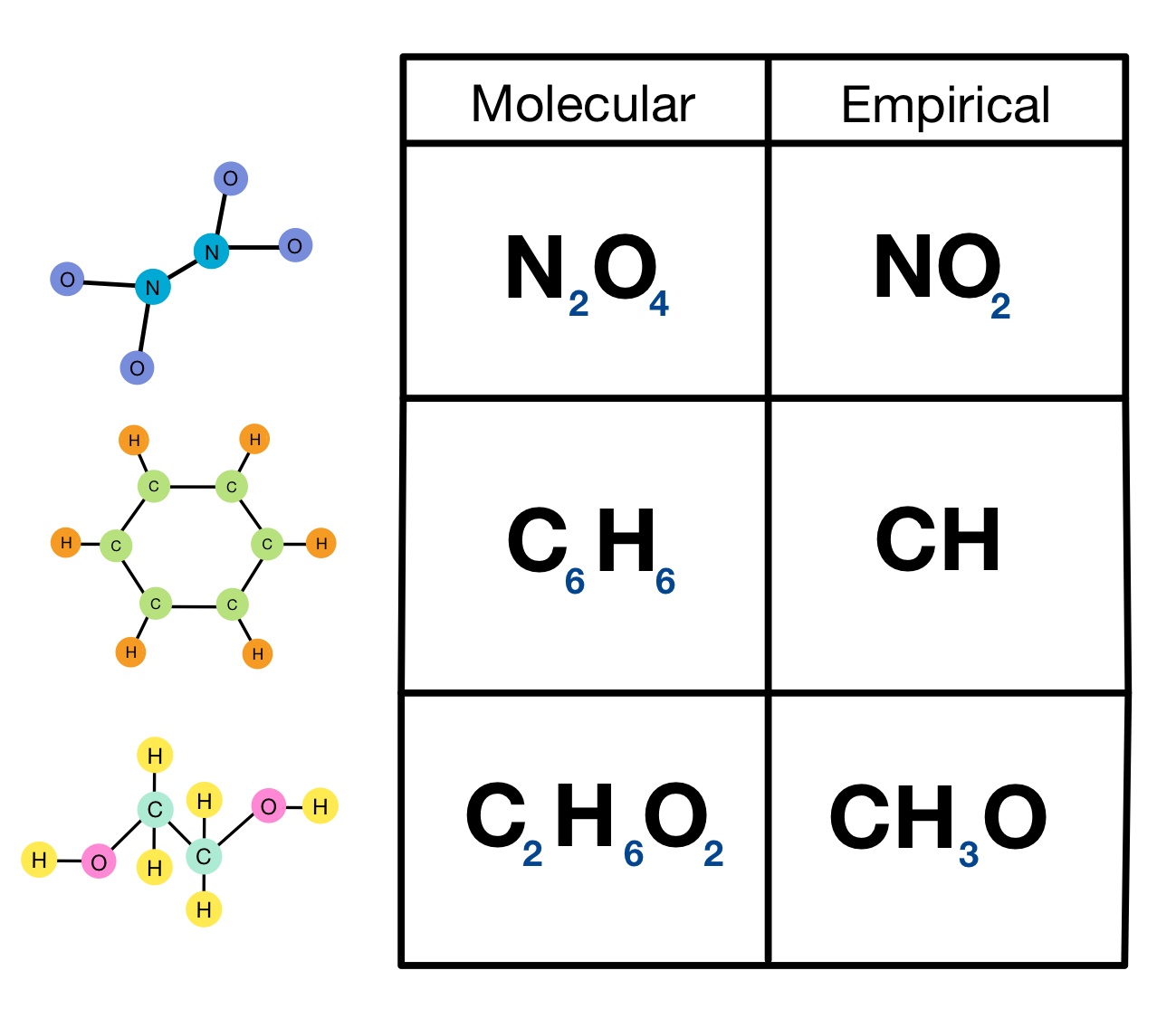
Empirical Vs Molecular Formula

Percent Composition Empirical Formula And Molecular Formula YouTube

Molecular Formula Worksheet Answer Key Naturalica

Question Video Determining An Empirical Formula From Percent

Stoichiometry Worksheet 3 Gram To Gram Calculations Answers Askworksheet
.PNG)
Percent Composition Worksheet 2

https:// chem.libretexts.org /Courses/Los_Angeles
WEB Skills to Develop Compute the percent composition of a compound Determine the empirical formula of a compound Determine the molecular formula of a compound In the previous section we discussed the relationship between the bulk mass of a substance and the number of atoms or molecules it contains moles

https:// kmacgill.com /chem/wp-content/uploads/2014/
WEB A compound has a molar mass of 86 g mol and has the percent composition by mass of 55 8 C 37 2 O and 7 0 H Determine the empirical formula and the molecular formula

https://www. teacherspayteachers.com /Product/
WEB This Science color by numbers activity includes 12 questions for students to practice calculating percent composition as well as finding empirical and molecular formulas Great for practice or assessment in your chemistry or physical science classroom

https://www. madison-schools.com /cms/lib/MS01001041
WEB Percent Composition and Molecular Formula Worksheet 1 Calculate the percent of nitrogen in urea NH2CONH2 2 Calculate the percentage of water in zinc sulfate heptahydrate 3 Calculate the percentage of oxygen in potassium chlorate 4 Calculate the percentage of tin in potassium stannate trihydrate K2SnO3 2H2O

https://www. auburn.wednet.edu /cms/lib03/WA01001938/
WEB Percent Composition and Molecular Formula Worksheet 1 What s the empirical formula of a molecule containing 65 5 carbon 5 5 hydrogen and 29 0 oxygen 2 If the molar mass of the compound in problem 1 is
WEB Apr 27 2023 0183 32 Determining the absolute numbers of atoms that compose a single molecule of a covalent compound requires knowledge of both its empirical formula and its molecular mass or molar mass These quantities may be determined experimentally by various measurement techniques WEB An empirical formula is the lowest common ratio between the elements in a compound The molecular formula is an ACTUAL real formula In order to calculate a molecular formula you need an empirical formula which can be given or calculated by YOU and the actual molecular mass of a compound
WEB Determine the empirical formula for each compound whose percentage composition is shown below 43 C and 57 O 40 3 K 26 7 Cr and 33 0 O 32 0 C 42 6 O 18 7 N and the remainder H 31 9 K 28 9 Cl and the remainder O 52 8 Sn 12 4 Fe 16 0 C and 18 8 N