Solubility Curve Practice Problems Worksheet 2 Answers Jun 9 2025 0183 32 Solubility is defined as the maximum quantity of a substance that can be dissolved in another It is the maximum amount of solute that can be dissolved in a solvent at equilibrium
5 days ago 0183 32 Solubility is a fundamental concept in chemistry that governs how substances interact forming the basis for countless natural phenomena and industrial processes It Solubility is the measure of how well a solute can dissolve in a solvent to form a solution It dictates how substances dissolve and interact impacting fields like pharmaceuticals
Solubility Curve Practice Problems Worksheet 2 Answers
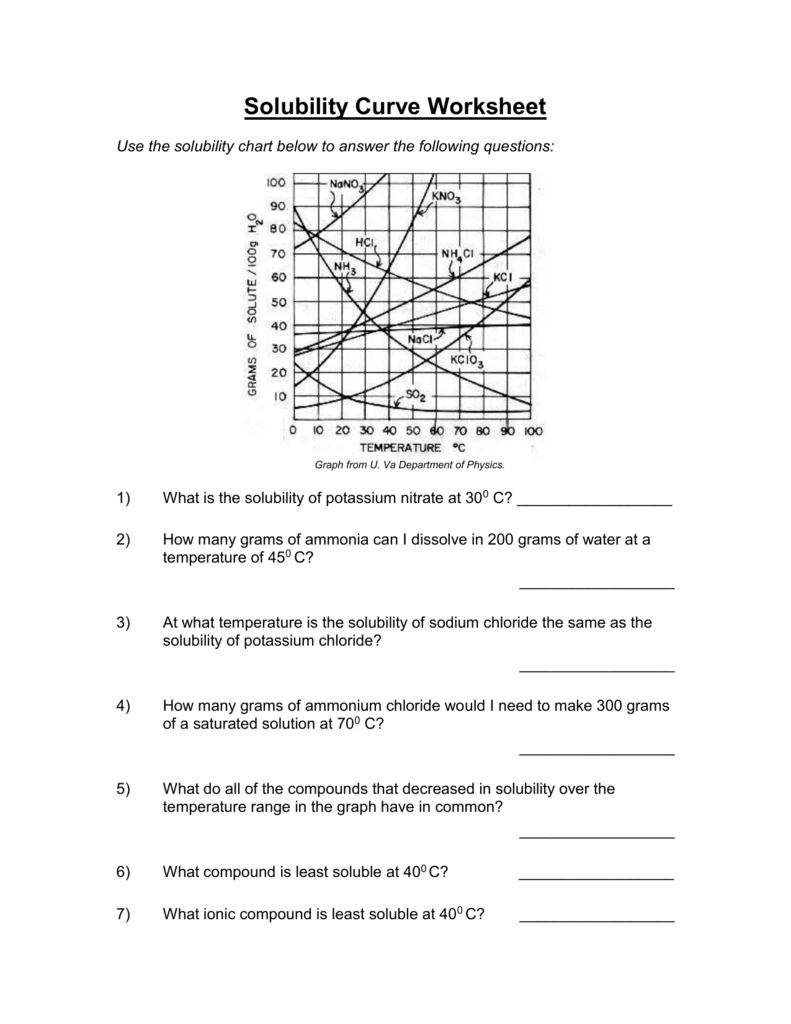 Solubility Curve Practice Problems Worksheet 2 Answers
Solubility Curve Practice Problems Worksheet 2 Answers
https://s3.studylib.net/store/data/007420914_1-5933bce2eddea2c581a28757a2e85e2d.png
Aug 13 2025 0183 32 Solubility is how well a solute dissolves in a solvent to form a solution including liquid in liquid solid in liquid and gas in liquid solutions
Templates are pre-designed documents or files that can be utilized for numerous purposes. They can conserve effort and time by supplying a ready-made format and design for developing various sort of material. Templates can be utilized for personal or professional jobs, such as resumes, invites, leaflets, newsletters, reports, presentations, and more.
Solubility Curve Practice Problems Worksheet 2 Answers

Solubility Curve Worksheet And Lab Answers Kidsworksheetfun
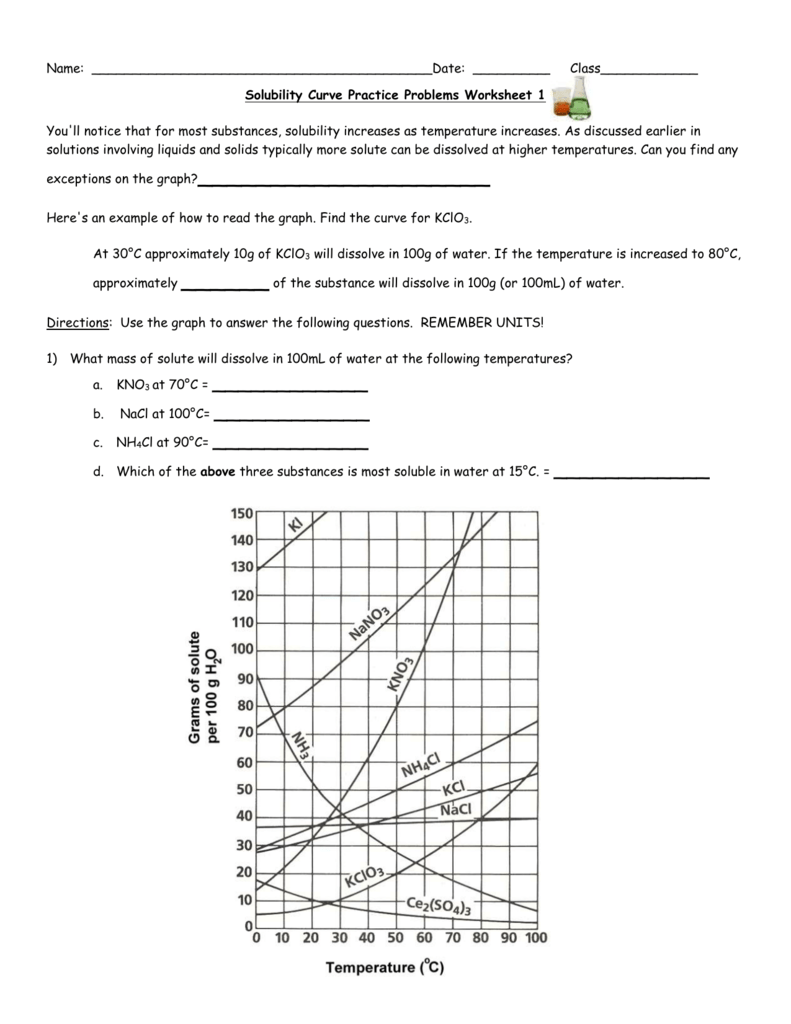
Solubility Curve Practice Problems Worksheet 1
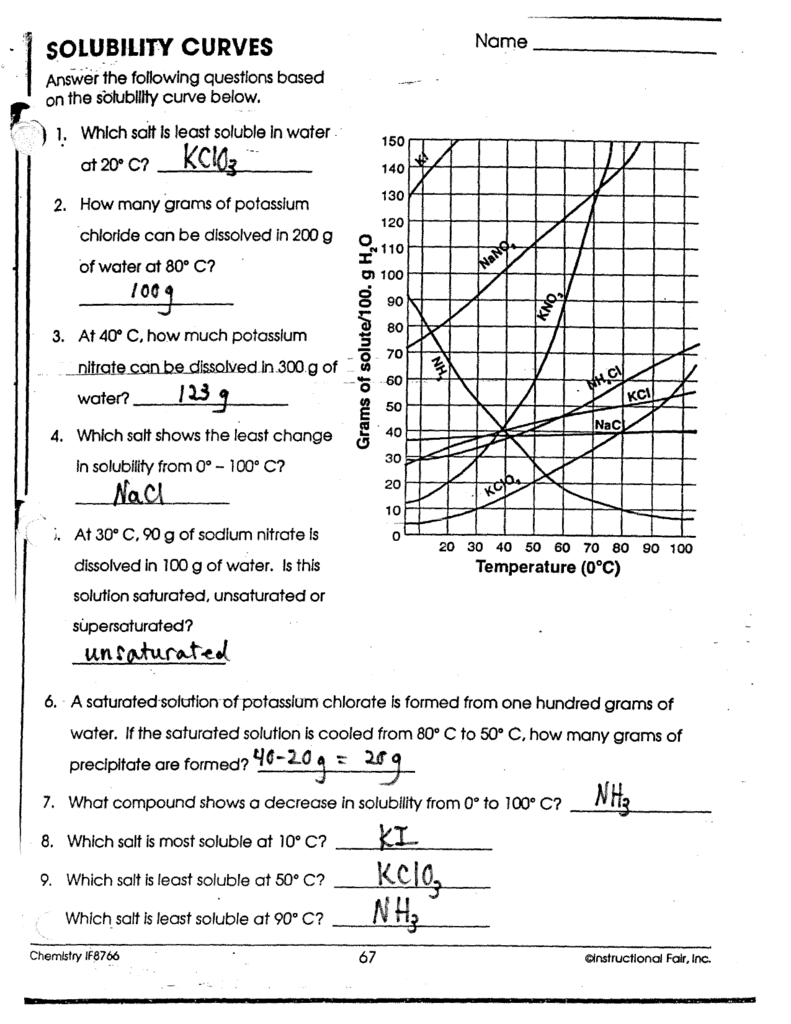
Solubility Curves
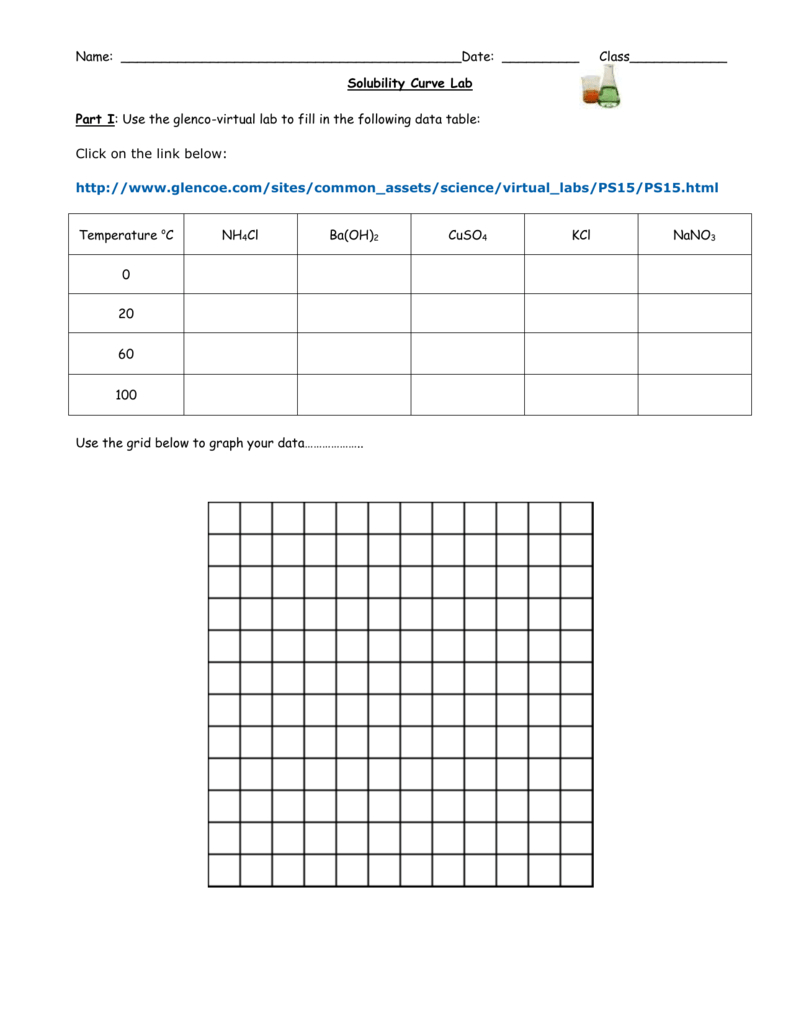
Solubility Curve Practice Problems Worksheet Db excel
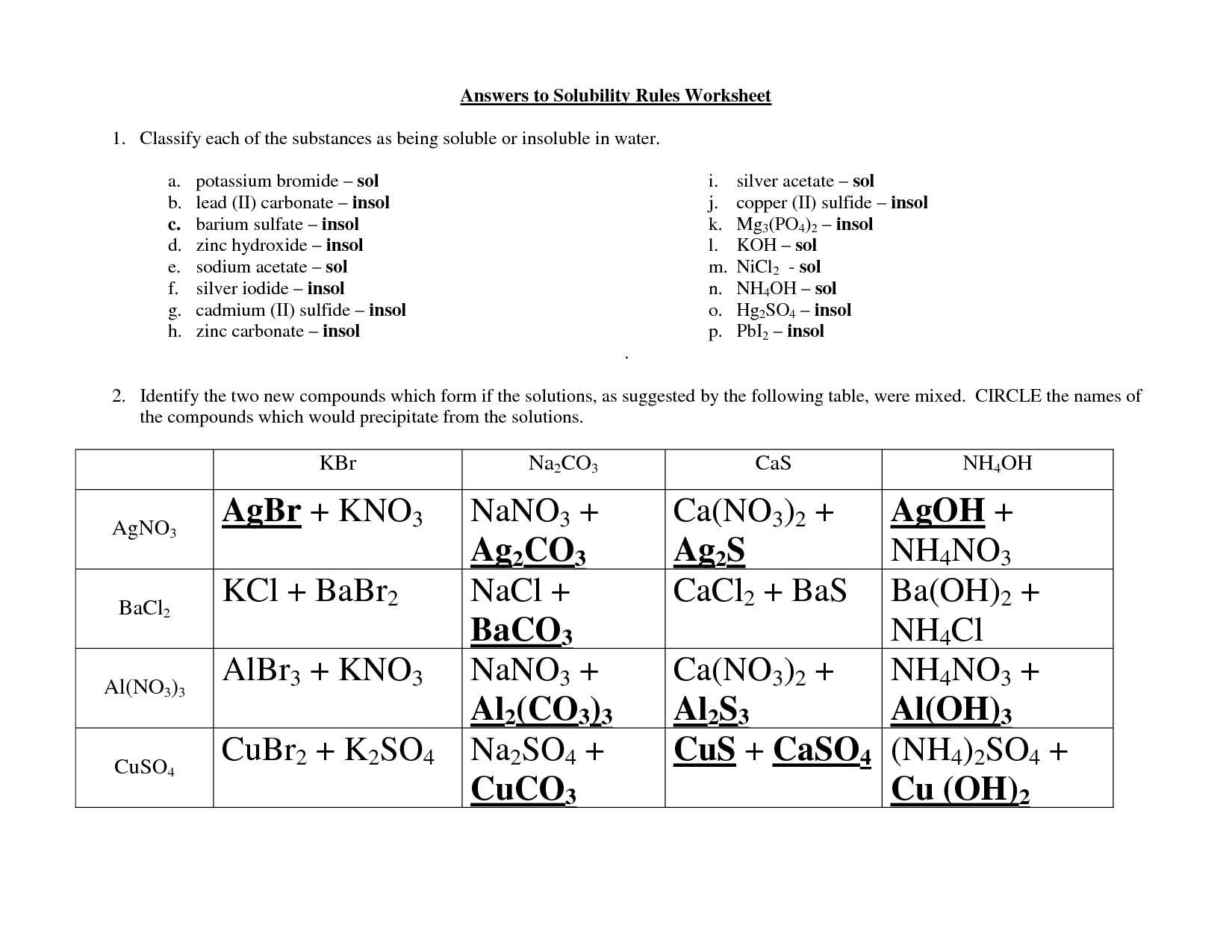
15 Best Images Of Who Rules Worksheet Divisibility Rules Worksheet
Solubility Curve Practice Problems Worksheet 1 Worksheet Template
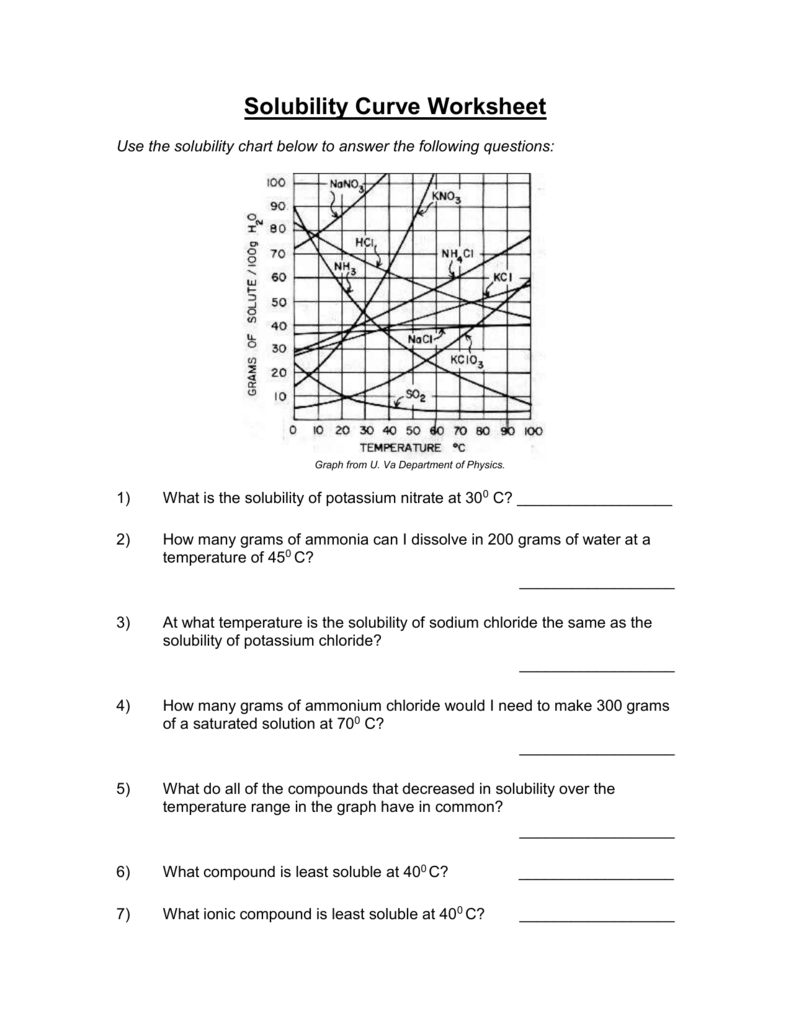
https://www.chemistrylearner.com › solubility
Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature The process through which a solute in its solid liquid or

https://byjus.com › chemistry › solubility
What is Solubility The maximum amount of solute that can dissolve in a known quantity of solvent at a certain temperature is its solubility A solution is a homogeneous mixture of one or

https://chem.libretexts.org › Bookshelves › Physical
Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium In such an equilibrium Le Chatelier s principle can be used to explain most of
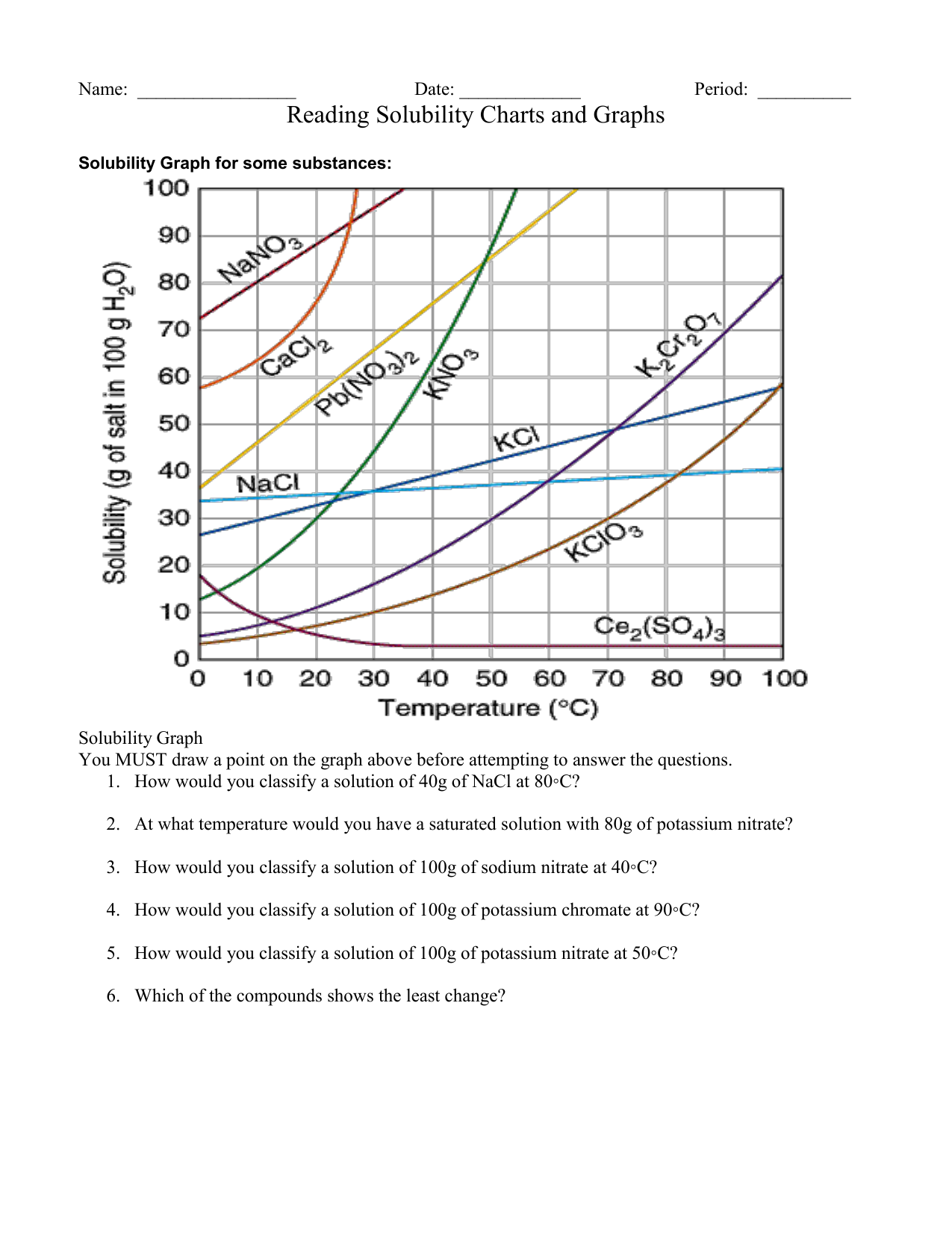
https://www.britannica.com › science › solubility-chemistry
Solubility of one fluid liquid or gas in another may be complete totally miscible e g methanol and water or partial oil and water dissolve only slightly

https://chemistrytalk.org › what-is-solubility
Solubility is the ability of a solute to dissolve in a solvent to form a solution This is the property that allows things like sugar molecules to dissolve in a cup of coffee
[desc-11] [desc-12]
[desc-13]