Solubility Polar Vs Nonpolar Solubility Rules There are a number of patterns in the data obtained from measuring the solubility of different salts These patterns form the basis for the rules outlined in the table below which can guide predictions of whether a given salt will dissolve in water
Solubility is defined as the upper limit of solute that can be dissolved in a given amount of solvent at equilibrium In such an equilibrium Le Chatelier s principle can be used to explain most of the main factors that affect solubility Solubility of one fluid liquid or gas in another may be complete totally miscible e g methanol and water or partial oil and water dissolve only slightly
Solubility Polar Vs Nonpolar
 Solubility Polar Vs Nonpolar
Solubility Polar Vs Nonpolar
https://sp-uploads.s3.amazonaws.com/uploads/services/4647090/20220814061845_62f893c5a69e8_solubility__polar_vs._nonpolar__worksheetpage0.jpg
Jun 9 2025 0183 32 Get the definition of solubility as the term is used in chemistry and learn about factors that affect it
Templates are pre-designed files or files that can be utilized for various functions. They can conserve effort and time by supplying a ready-made format and design for creating different kinds of material. Templates can be utilized for personal or professional jobs, such as resumes, invites, leaflets, newsletters, reports, presentations, and more.
Solubility Polar Vs Nonpolar

Solubility Polar Vs Nonpolar YouTube

Worksheet Polarity Of Bonds Answers Determining Molecular Polarity
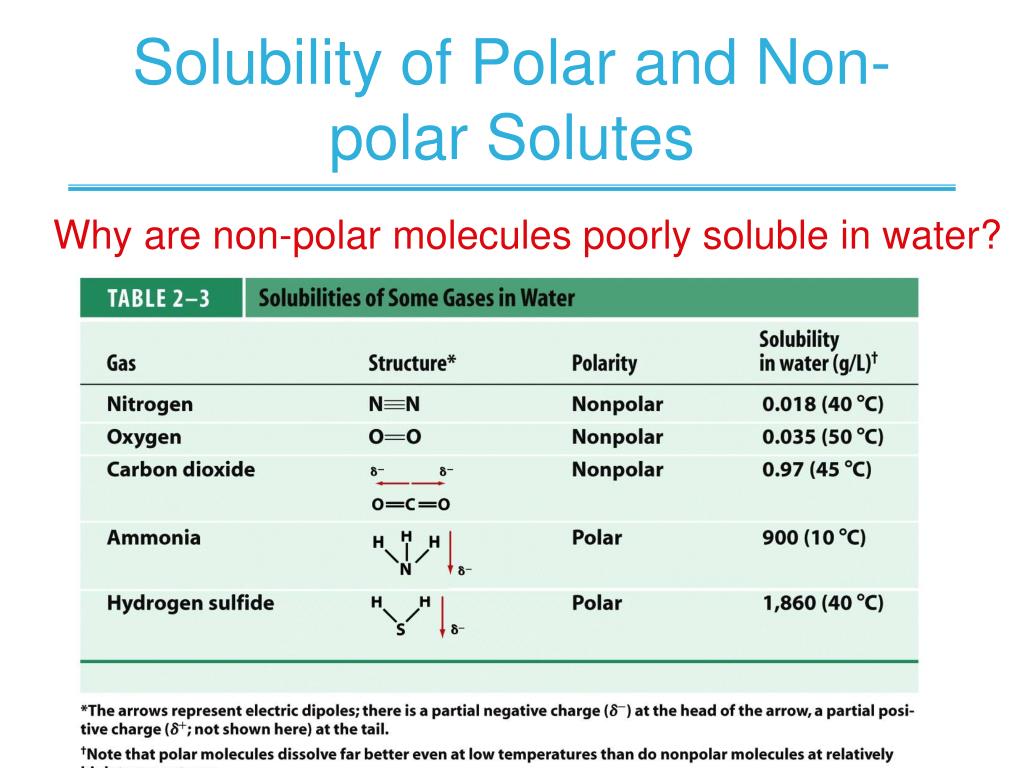
PPT CHAPTER 2 Water And Aqueous Solutions PowerPoint Presentation
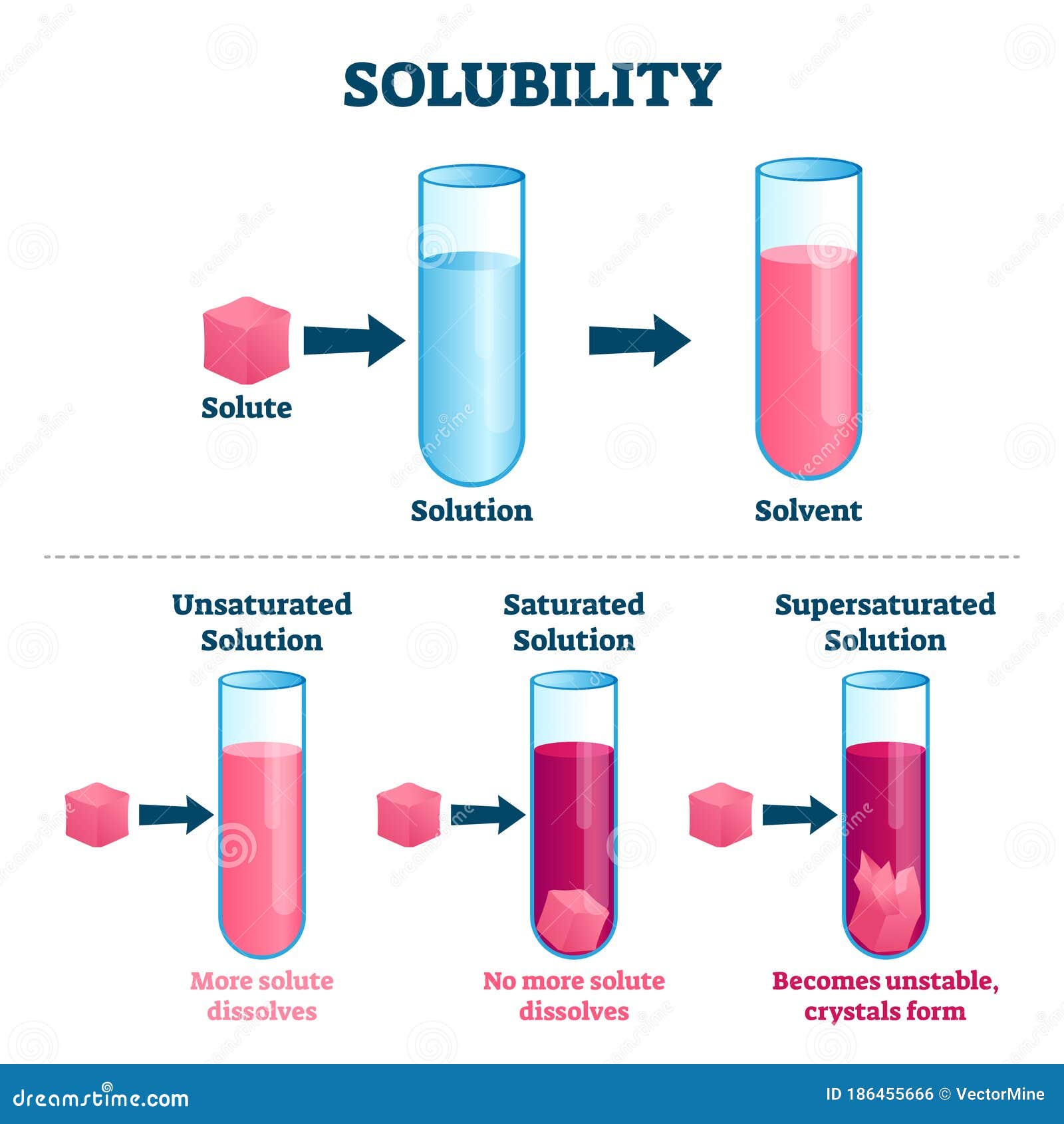
Illustration De Vecteur De Solubilit Plan Marqu De Solvant Et De

Hoy s Solubility Parameters For Nonpolar Vs Polar Solvents Download
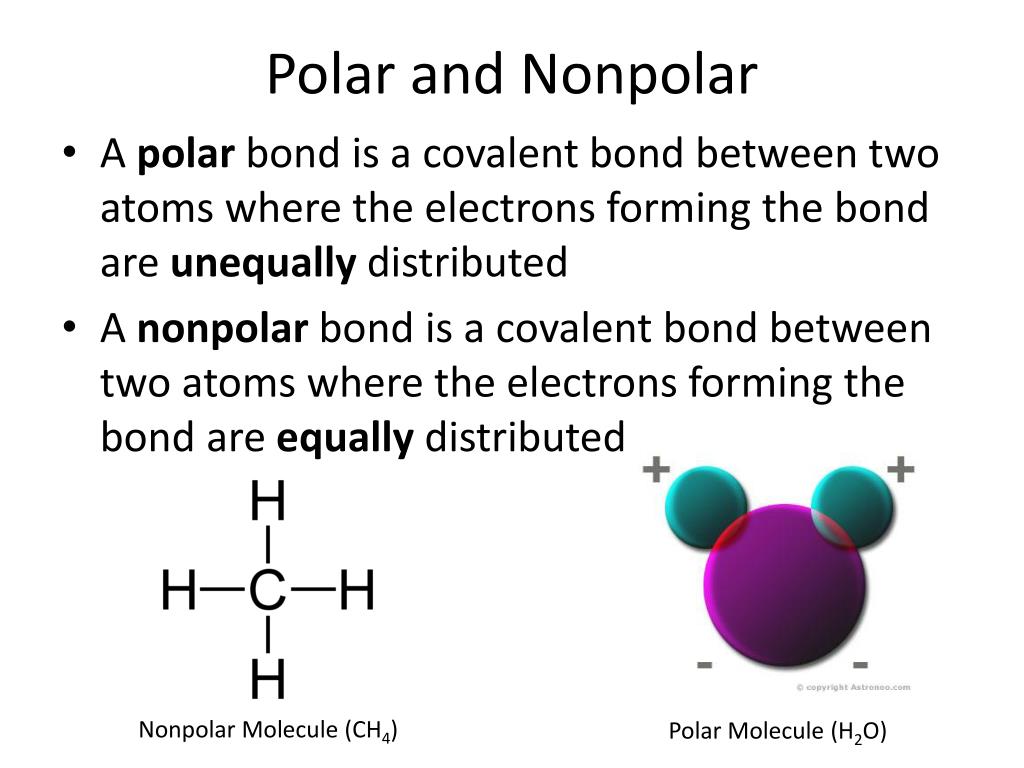
PPT Solubility PowerPoint Presentation Free Download ID 2516429

https://chemistrytalk.org › what-is-solubility
Factors That Affect Solubility Temperature One of the factors that may affect a solutes solubility is the variation in temperature If the temperature is changed we can increase the solubility of a substance A sparingly solid liquid can be dissolved completely by increasing the temperature making that substance soluble at the given temperature

https://www.samaterials.fr › blog › solubility-in-chemistry.html
La solubilit 233 est une mesure de la quantit 233 de solut 233 qui peut se dissoudre dans une quantit 233 donn 233 e de solvant pour former une solution stable La solubilit 233 varie en fonction de la temp 233 rature de la pression et de la nature du solut 233 et du solvant

https://www.greelane.com › fr › science-technologie
Jan 22 2020 0183 32 Obtenez la d 233 finition de la solubilit 233 comme le terme est utilis 233 en chimie et d 233 couvrez les facteurs qui l affectent
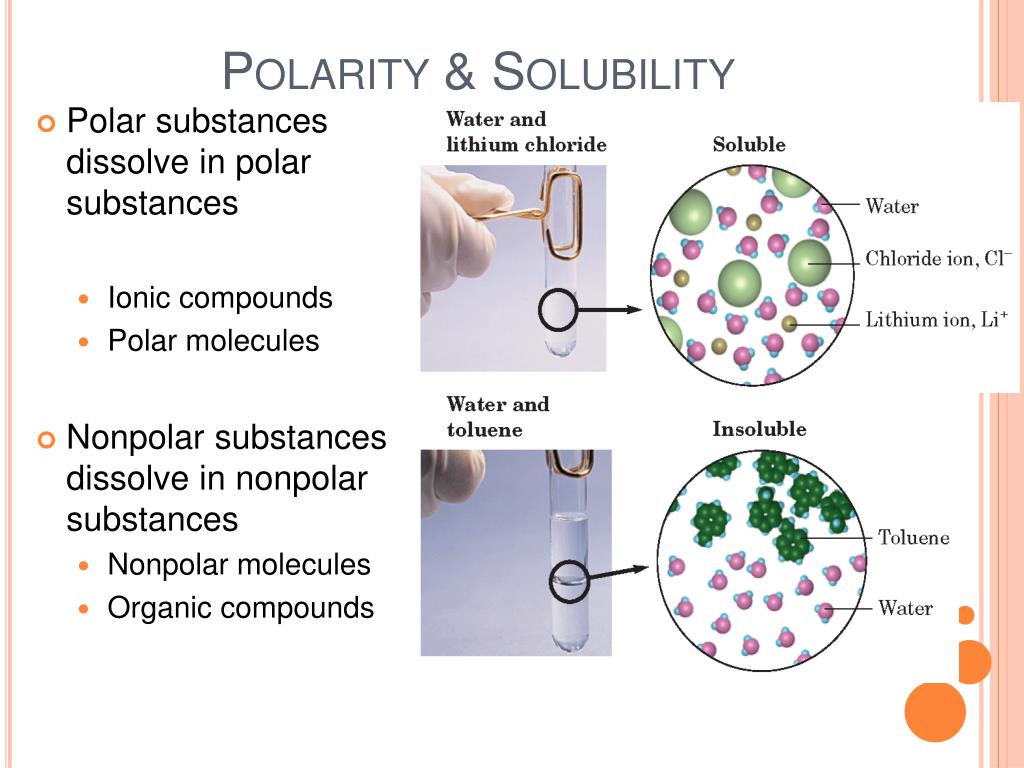
https://www.chemistrylearner.com › solubility
What is solubility and what are its units What two factors affect it the most Check out a few examples Learn water solubility miscibility and solubility curves

https://biologyinsights.com › what-is-solubility
Aug 18 2025 0183 32 Solubility is a fundamental concept in chemistry that governs how substances interact forming the basis for countless natural phenomena and industrial processes It describes the capacity of a substance to mix uniformly with another creating a homogeneous blend What Solubility Really Means Solubility refers to the ability of a substance known as the solute to
[desc-11] [desc-12]
[desc-13]