Stoichiometry Grams To Grams Calculator The Reaction Stoichiometry Calculator allows you to balance a chemical equation and establish the relationship between the amounts of reactants and products of the reaction To use the Stoichiometry Salculator you just need to write the chemical equation in the input field and then press the calculate button
Convert grams to moles and moles to grams This grams to moles moles to grams calculator converts between moles and grams using a formula of a substance In many chemistry problems you need to convert grams to moles or moles to grams The calculator below calculates the mass of the substance in grams or the quantity of the Share a link to this widget More Embed this widget 187
Stoichiometry Grams To Grams Calculator
 Stoichiometry Grams To Grams Calculator
Stoichiometry Grams To Grams Calculator
https://i.ytimg.com/vi/Bj3fr5pEn7U/maxresdefault.jpg
Stoichiometry Calculator Utilize our online free Stoichiometry Calculator and solve the chemical stoichiometry equation Provide your input equation and reactants or products mass moles in the input box and press the calculate button to obtain the stoichiometry values of the balanced given equation as output within a short span of time
Pre-crafted templates provide a time-saving service for producing a varied series of files and files. These pre-designed formats and designs can be utilized for different individual and expert projects, including resumes, invites, flyers, newsletters, reports, discussions, and more, enhancing the content development procedure.
Stoichiometry Grams To Grams Calculator

Stoichiometry Grams To Moles Moles To Grams Or Moles To Moles 1 Or

Stoichiometry Mole To Mole Grams To Grams Mole Ratio Practice
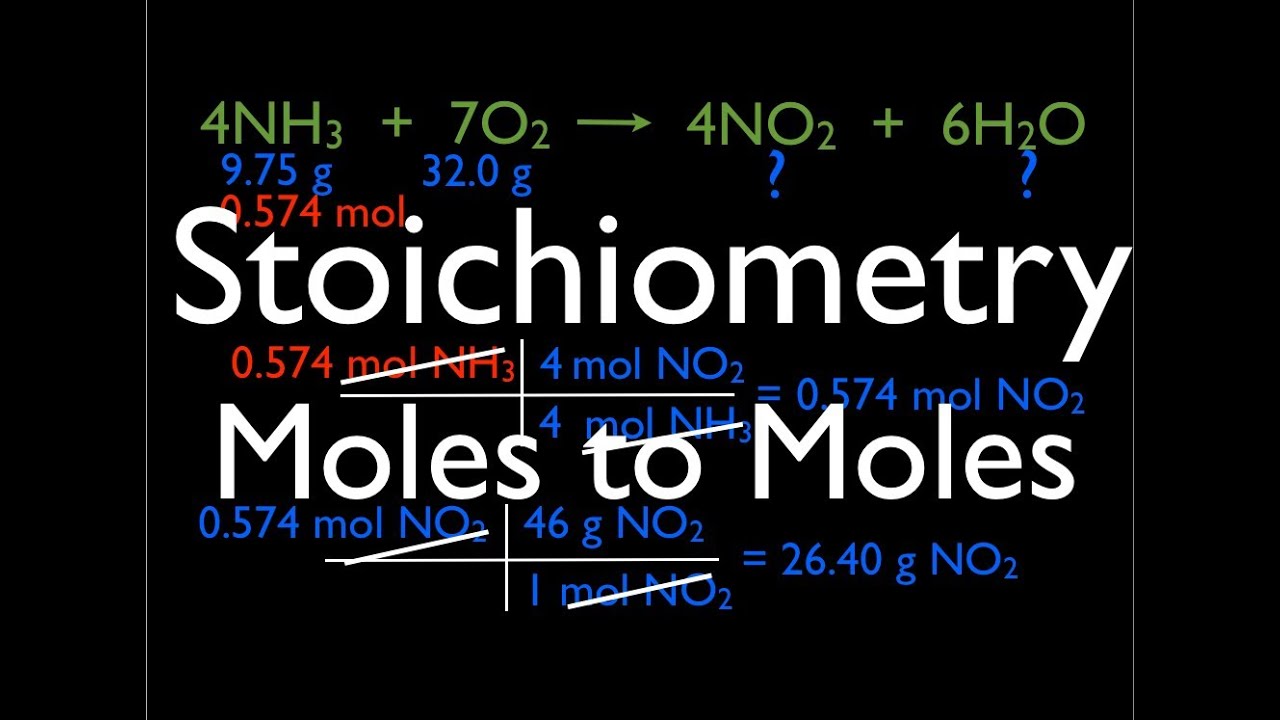
Stoichiometry Moles To Moles YouTube
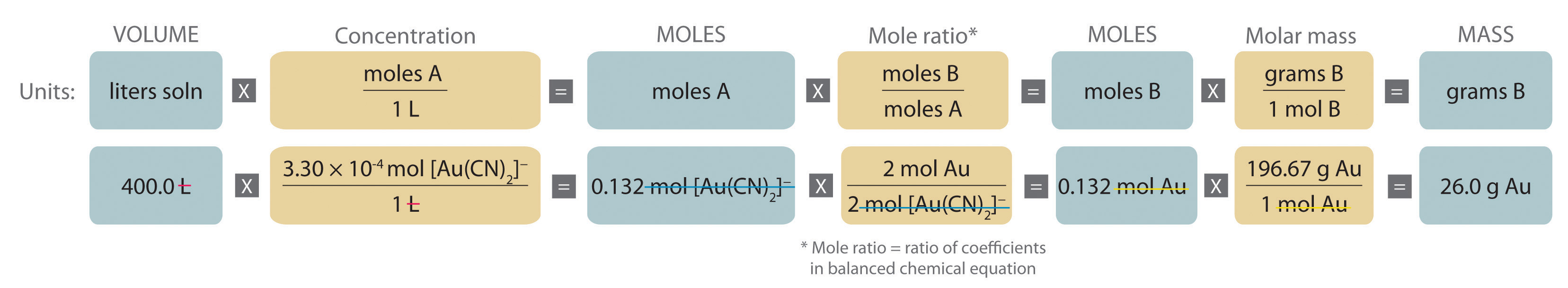
Stoichiometry Of Reactions In Solution

Stoichiometry grams To Grams YouTube

Moles To Grams Stoichiometry YouTube

https://ezcalc.me/stoichiometry-calculator
This online Stoichiometry Calculator finds the stoichiometric coefficients to balance a given chemical equation and computes amounts of the reactants and products of the reaction both in moles and grams

https://www.chemicalaid.com/tools/reactionstoichiometry.php
You now know the number of moles of each substance needed in a theoretical perfect reaction If you want to know the number of grams needed of each substance you can multiply by the molar mass of each substance NaOH 2 10 mol 39 997 g mol 83 9937g Mg OH 2 1 05 mol 58 319 g mol 61 23495g

https://chem.libretexts.org/Courses/Bellarmine
Flowchart of steps in stoichiometric calculations Step 1 grams of A is converted to moles by multiplying by the inverse of the molar mass Step 2 moles of A is converted to moles of B by multiplying by the molar ratio Step 3 moles of B is converted to grams of B by the molar mass

https://studyqueries.com/stoichiometry-calculator
The Stoichiometry Calculator is a free online tool that displays a balanced equation for a chemical equation STUDYQUERIES s online stoichiometry calculator tool makes calculations faster and it displays the balanced equation in a fraction of a second How to Use Stoichiometry Calculator Here is how to use the Stoichiometry calculator

https://www.youtube.com/watch?v=bltnuzbs2JA
Feb 15 2013 0183 32 Chemical Reactions 9 of 11 Stoichiometry Grams to Grams Shows how to use stoichiometry to determine the grams of the other substances in the chemical equation if you are given the
Mar 28 2018 0183 32 Stoichiometry Converting Grams to Grams How many grams of Ca OH 2 are needed to react with 41 2 g of H3PO4 The equation is 2 H3PO4 3 Ca OH 2 Ca3 PO4 2 6 H2O more How many Mass to mass conversion from a chemical equation A chemical equation gives a mole to mole conversion factor If the given substance is in grams and the desired substance is also in grams then two additional conversion factors based on the molar masses are needed That is the following conversions are needed
Steps to getting this answer Since you cannot calculate from grams of reactant to grams of products you must convert from grams of C 3H 8 to moles of C 3H 8 then from moles of C 3H 8 to moles of H 2O Then convert from moles of H 2O to grams of H 2O Step 1 200 g C 3H 8 is equal to 4 54 mol C 3H 8