The Mole Volume Relationship Worksheet Answers i Mole fraction It is the ratio of the number of moles of a particular component to the total number of moles of all the components in the mixture It is denoted by symbol
Hence if P A is the vapor pressure of the solvent x A is its mole fraction and P A 0 is the vapor pressure of the pure solvent then by Raoult s law the relation will come out to be P A x A P In this chapter laws of chemical combination Dalton s atomic theory mole concept empirical and molecular formula stoichiometry and its calculations are discussed Download NCERT
The Mole Volume Relationship Worksheet Answers
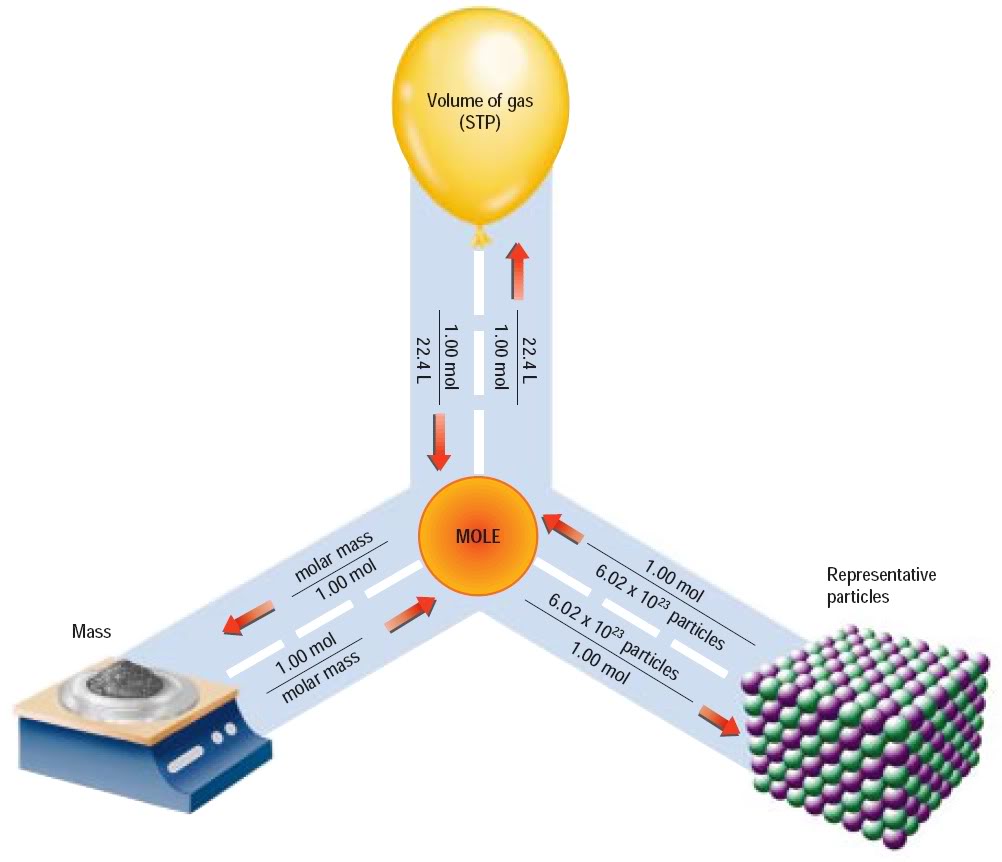 The Mole Volume Relationship Worksheet Answers
The Mole Volume Relationship Worksheet Answers
http://1.bp.blogspot.com/-hrCm30OOPVg/TuUGIPFphXI/AAAAAAAAAFE/ePAPtZAIoK0/s1600/Mole+Relationships.jpg
How many moles of electrons weight one kilogram Mass of electron 9 1 10 31 kg avogadro number 6 023 10 23
Templates are pre-designed files or files that can be used for numerous purposes. They can conserve effort and time by offering a ready-made format and layout for developing various type of material. Templates can be used for individual or professional jobs, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
The Mole Volume Relationship Worksheet Answers
Graphing Proportional Relationship Worksheets WorksheetsGO

Mole To Liter Conversions Mole To Volume Detailed Examples And
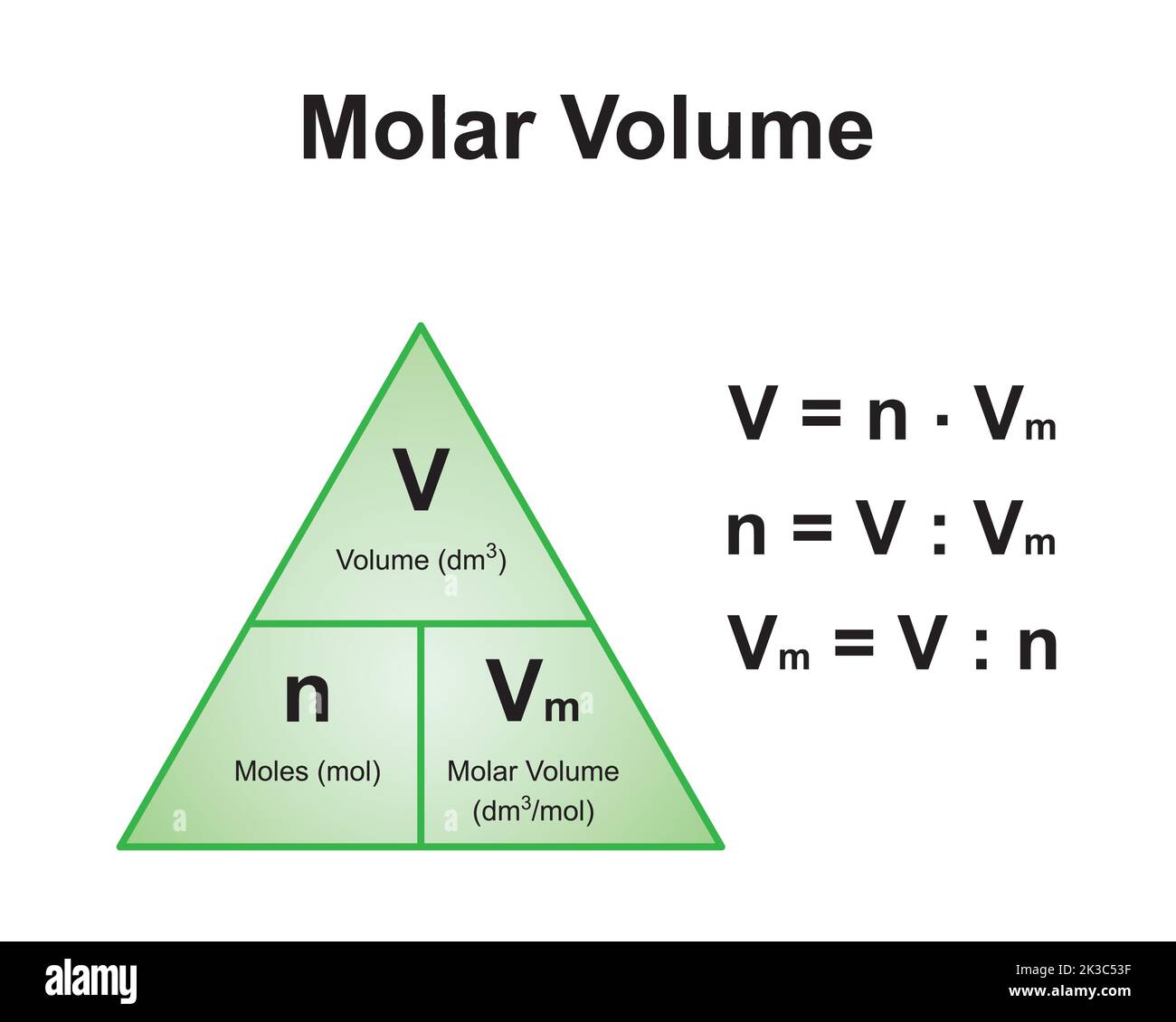
Scientific Designing Of The Mole And Molar Volume Formula Triangle
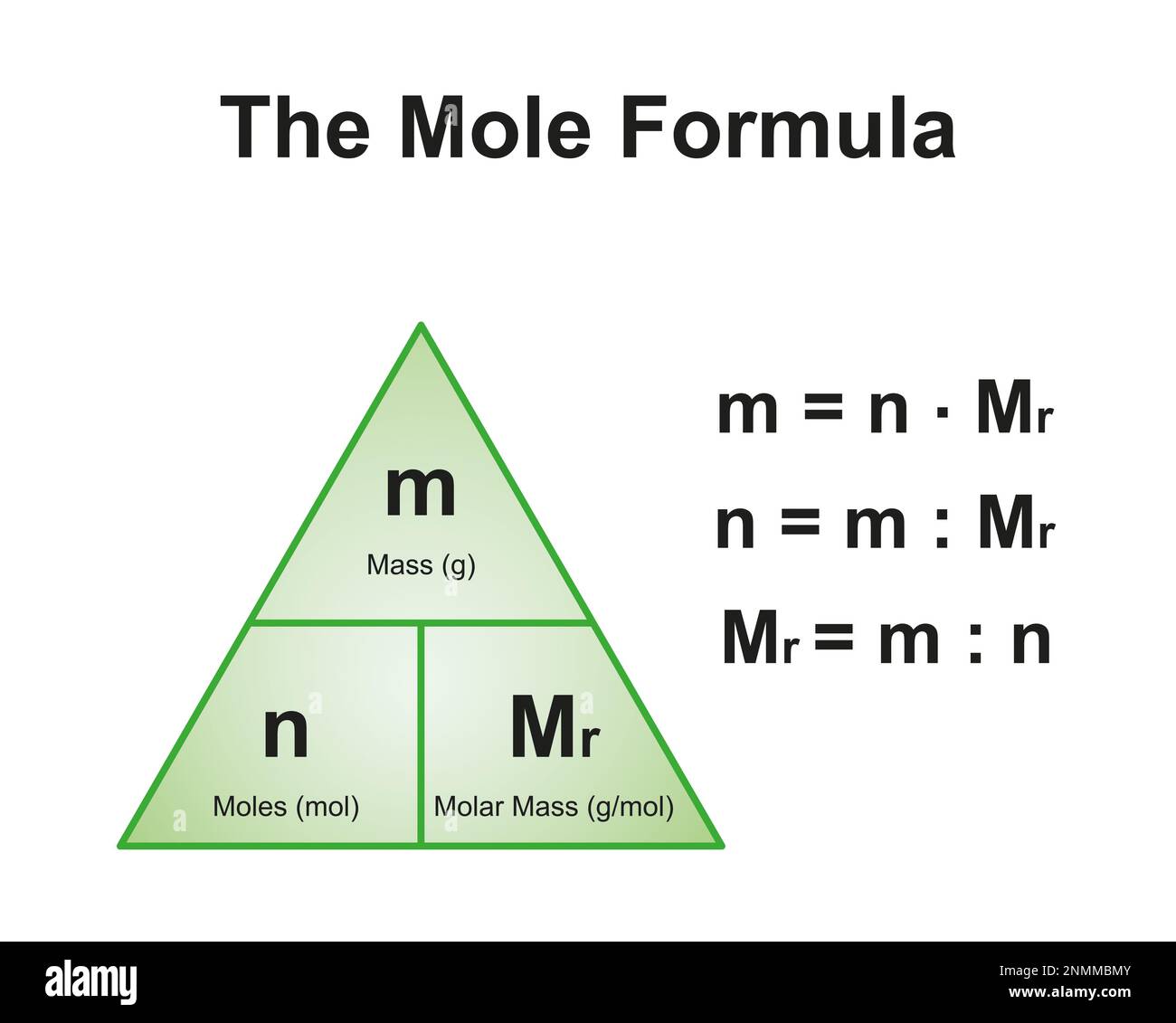
Mole Formula Illustration Stock Photo Alamy
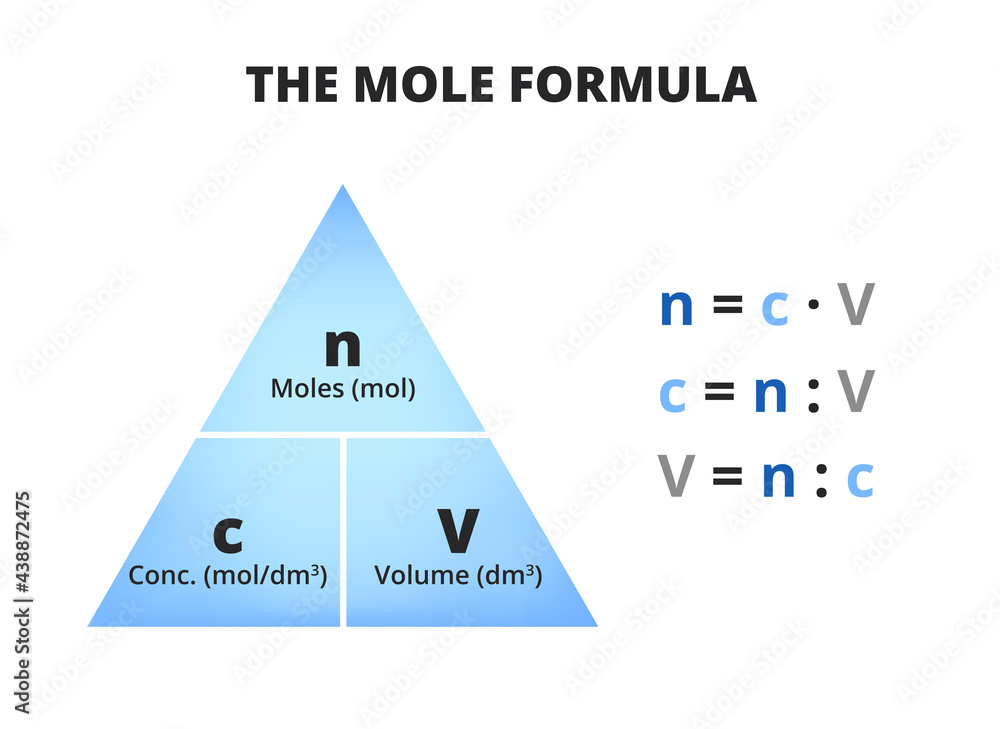
The Mole And Concentration Formula Triangle Or Pyramid Isolated On A
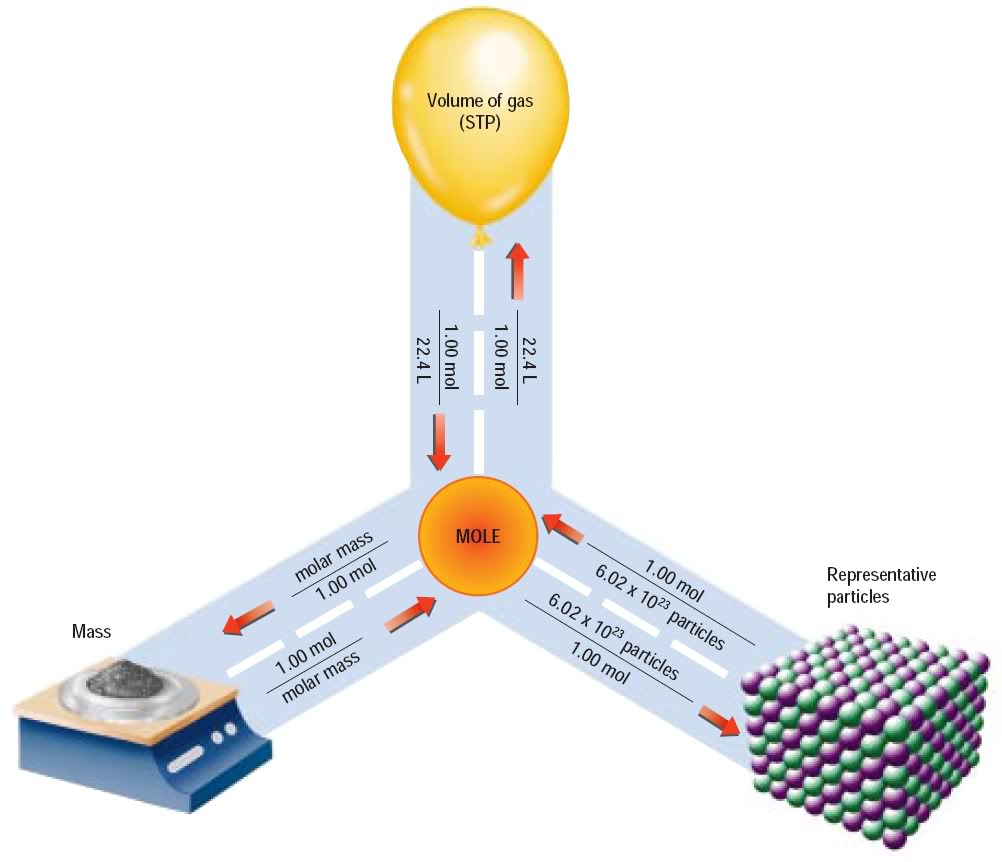
Grade 10 Physical Science Stoichiometry
https://www.toppr.com › guides › chemistry › some-basic-concepts-of-ch…
We define a mole as the number equal to Avogadro s number This is just like you say dozen is equivalent to 12 a score is 20 and a century is 100 In other words a mole is a unit that we use

https://www.toppr.com › guides › chemistry › atoms-and-molecules › mol…
Ans The molar quantity of Ar is available and should be used for deriving the corresponding mass in terms of grams Note that the amount of Ar is mentioned less than 1 mole hence the mass

https://www.toppr.com › guides › physics › atomic-and-molecular-structu…
One mole of any substance atom molecule etc is equal to its atomic mass or molecular mass expressed in grams The number of particles present in one mole of any substance atom or

https://www.toppr.com › ask › question › define-mole-fraction
Define mole fraction A solution of sucrose in water is labelled as 20 w w What would be the mole fraction of each component in the solution

https://www.toppr.com › ask › question
The vapour pressure of a pure liquid at 25 176 C is 100 mm Hg Calculate the relative lowering vapour pressure if the mole fraction of solvent in solution is 0 8 p 186 PS Xsolute 100 15 4802 Loo
[desc-11] [desc-12]
[desc-13]