Theoretical And Percent Yield Worksheet Pdf Limiting Reactant Theoretical amp Percent Yield Practice 1 When copper II chloride reacts with sodium nitrate copper II nitrate and sodium chloride are formed a Balance the equation for
Practicing percentage yield calculations 1 Aluminium reacts with hydrochloric acid to form hydrogen gas and aluminium chloride as shown in the reaction shown below You are given Determine the percentage yield obtained if 1280 mol of carbon is consumed and 622 mol of CaSiO3 is produced
Theoretical And Percent Yield Worksheet Pdf

https://imgv2-1-f.scribdassets.com/img/document/338147515/original/e552425bd2/1628687577?v=1
Solve stoichiometric problems from a balanced chemical equation Differentiate between the actual yield and theoretical yield of a chemical reaction Calculate the theoretical yield from a
Pre-crafted templates provide a time-saving option for developing a diverse variety of files and files. These pre-designed formats and layouts can be made use of for numerous individual and expert projects, consisting of resumes, invites, flyers, newsletters, reports, discussions, and more, simplifying the content development procedure.
Theoretical And Percent Yield Worksheet Pdf

Limiting Reagent And Percent Yield Worksheets
Percent Yield Problems

Percent Yield Worksheet

Theoretical And Percent Yield YouTube

141 Limiting Reactant Worksheet Key Limiting Reactant Theoretical

Limiting Reactant And Percent Yield Worksheet PDF

https://www.tsfx.edu.au › resources
Percent Yield Calculations Practice Problems 1 A reaction with a 183 calculated yield of 9 23 g produced 7 89 g of product What is the percent yield for this reaction 2 5 96 g of ammonia
https://midtgard.weebly.com › uploads
Stoichiometry Percent Yield Worksheet Key 1 Write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate

http://teachnlearnchem.com › Keys Worksheets › KEYS S…
X g excess x g theoretical yield If you must produce 700 g of ammonia what mass of nitrogen should you use in the reaction assuming that the percent yield of this reaction is 70

https://colemangenchem.weebly.com › uploads ›
Theoretical and Percent Yield Worksheet 1 Write the equations for calculating yield and error in the boxes below 2 What does a yield tell you 3 What does a error tell you

https://www.csun.edu › LIMITREG.pdf
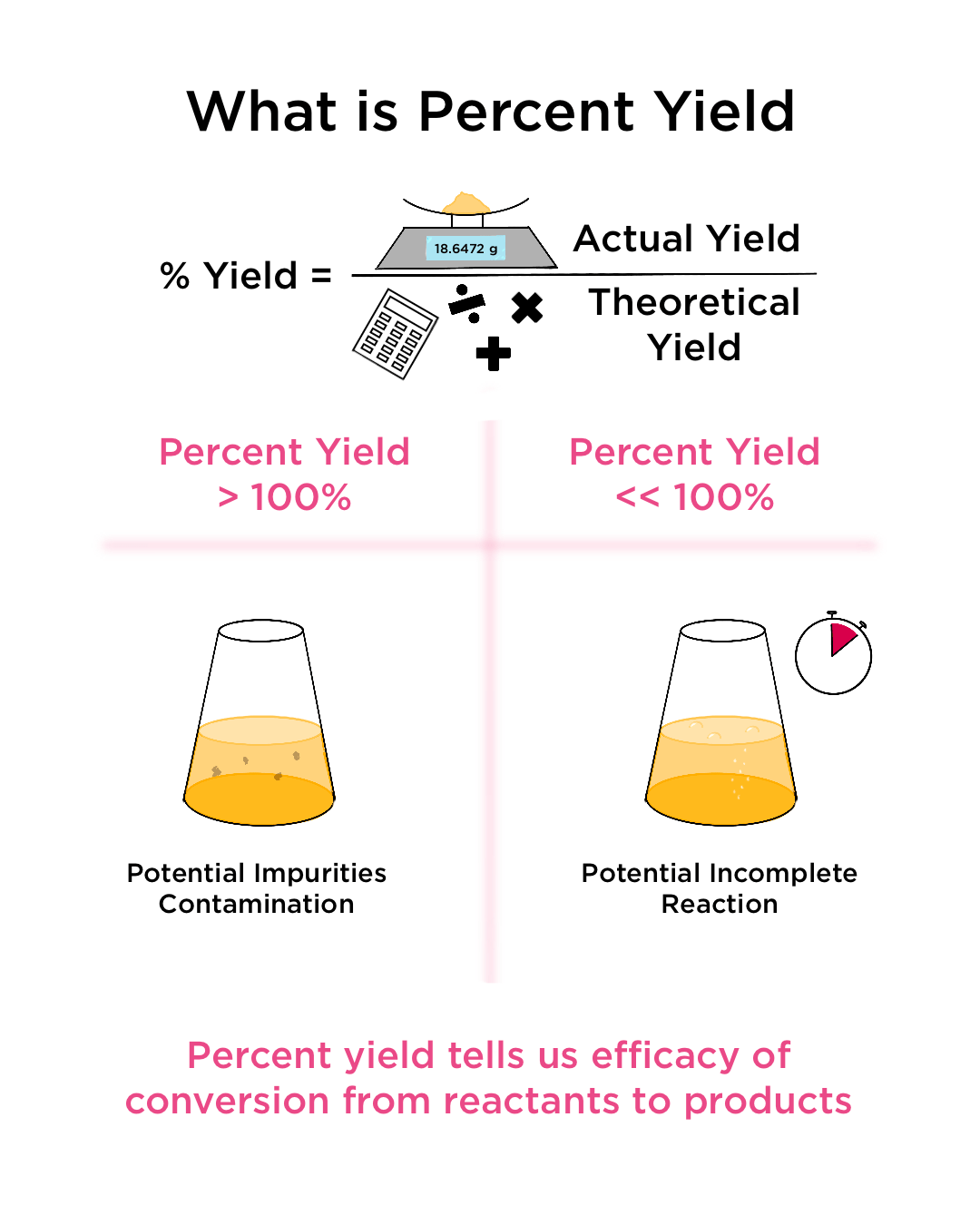
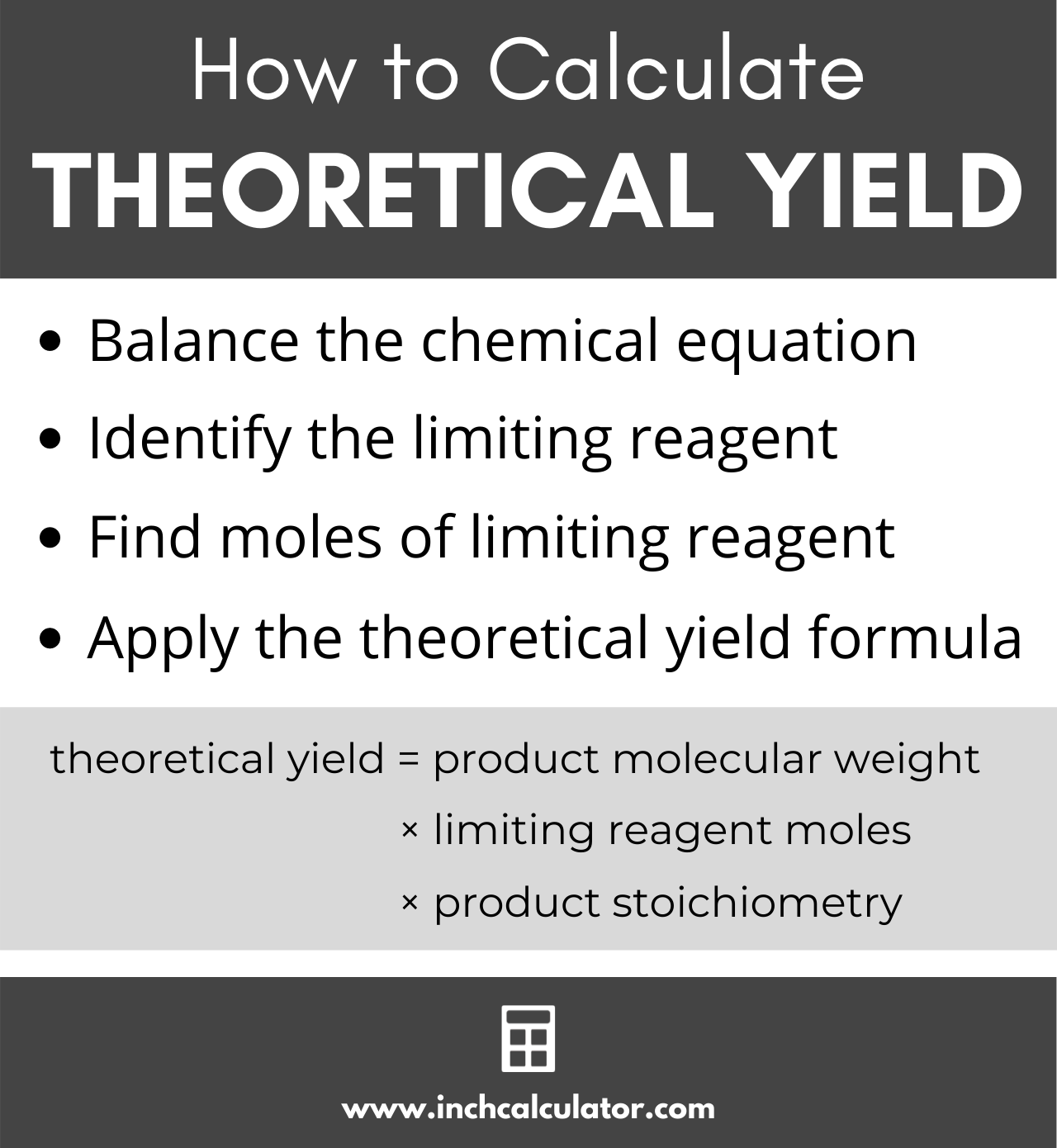
The theoretical yield is the amount of the product in g formed from the limiting reagent From the moles of limiting reagent available calculate the grams of product that is theoretically possible
Percent Yield Calculation Answers 1 Balance this equation and state which of the six types of reaction is taking place 1 Mg 2 HNO 3 1 Mg NO 3 2 1 H 2 Type of reaction single What is my percent yield of titanium II oxide if I start with 20 grams of titanium II sulfide and my actual yield of titanium II oxide is 22 grams 137 5 theoretical yield is 16 0 grams
Theoretical and Percent Yield Worksheet Hon chem coleman 1 Write the equations for calculating yield and error in the boxes below 2 What does a yield tell you 3 What