What Are Intensive And Extensive Properties In Thermodynamics Summary An extensive property is a property that depends on the amount of matter in a sample Mass and volume are examples of extensive properties An intensive property is a property of matter that depends only on the type of matter in
Intensive properties are independent of the mass of a system Pressure P P temperature T T specific volume v v specific internal energy u u specific enthalpy h h and specific entropy s s are intensive properties Let us consider a There is a useful and important distinction in thermodynamics between extensive or capacitive and intensive quantities Extensive quantities are those that depend upon the amount of material Examples would include the volume or the heat capacity of a body
What Are Intensive And Extensive Properties In Thermodynamics
 What Are Intensive And Extensive Properties In Thermodynamics
What Are Intensive And Extensive Properties In Thermodynamics
https://pressbooks.bccampus.ca/thermo1/wp-content/uploads/sites/499/2021/07/intensive-and-extensive-properties.png
Properties that are not proportional to the sample size are called intensive properties Examples of intensive properties are pressure P temperature T density heat capacities Cv Cp and rms velocity vrms When a sample is in a state of equilibrium the values of all intensive properties will be uniform throughout the sample
Templates are pre-designed documents or files that can be used for different functions. They can save time and effort by supplying a ready-made format and design for developing different sort of material. Templates can be used for individual or professional projects, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
What Are Intensive And Extensive Properties In Thermodynamics
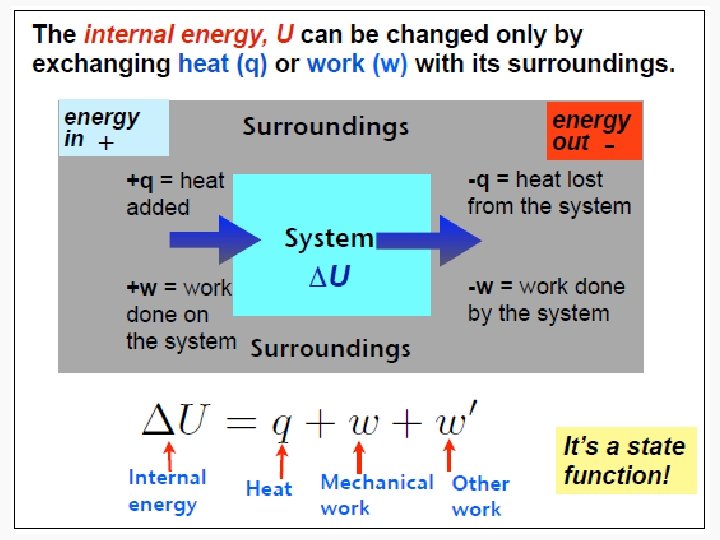
Thermodynamics Intensive And Extensive Properties Intensive Properties
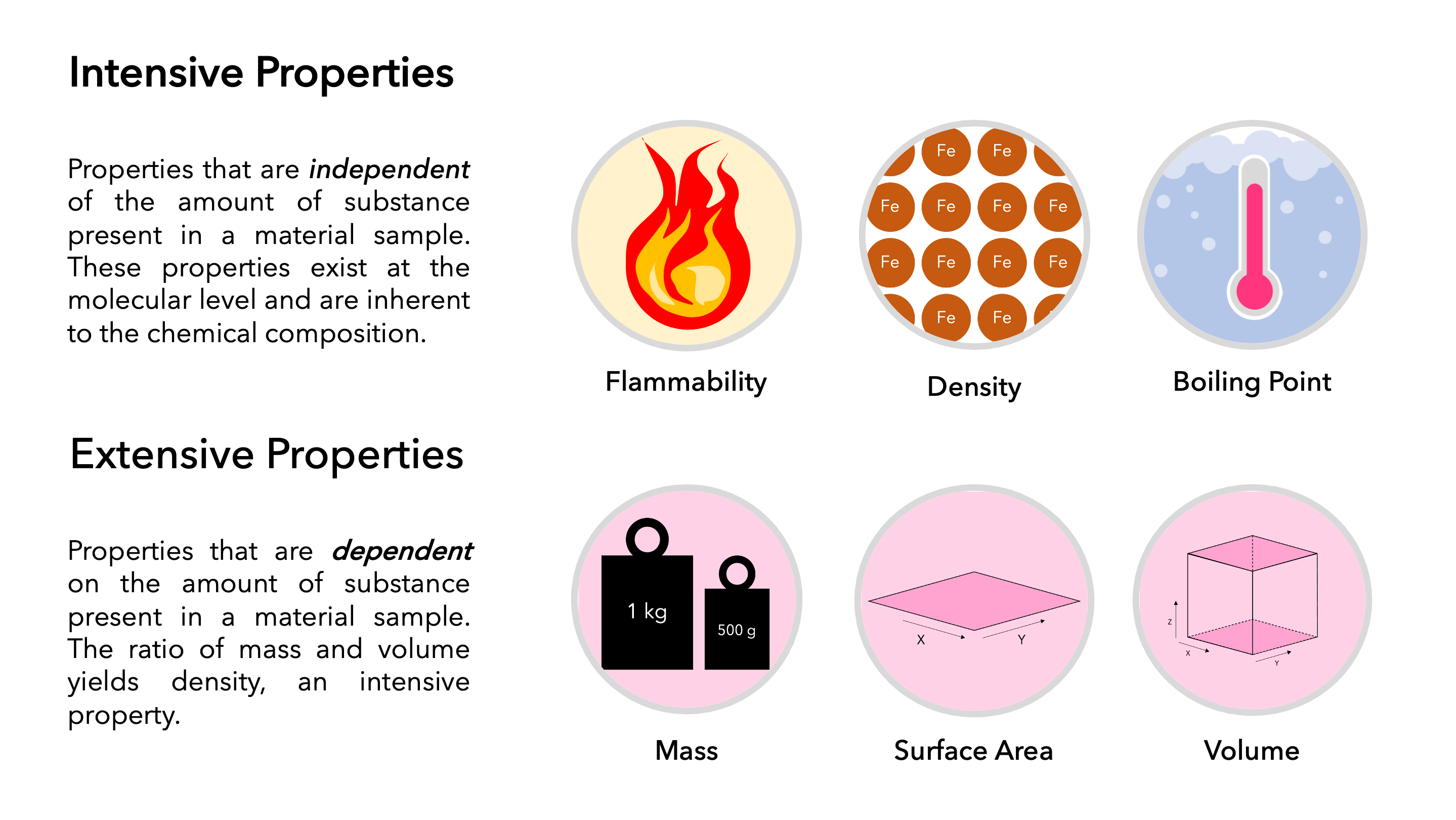
Describe The Difference Between An Extensive Property And Intensive
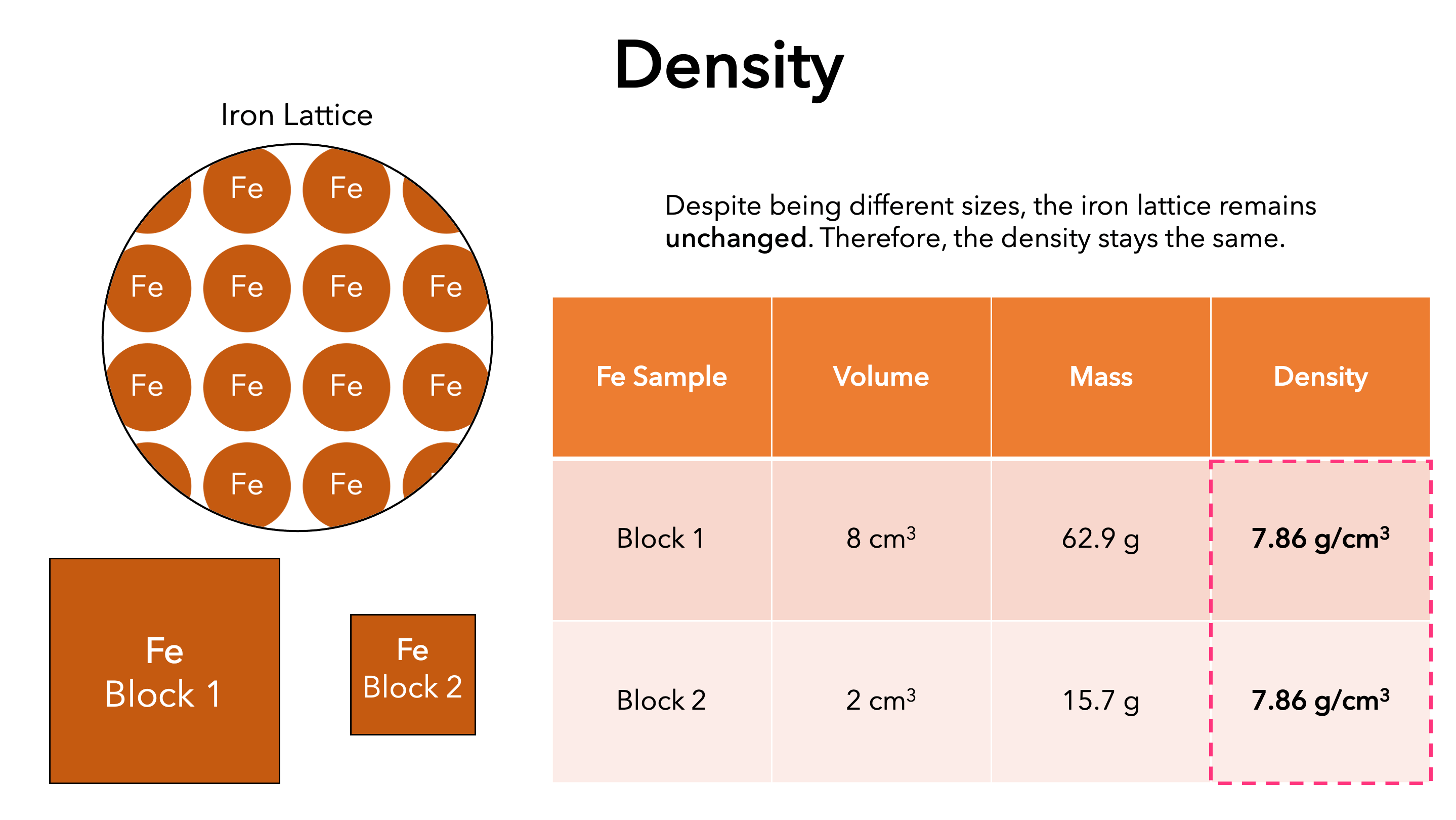
Extensive Vs Intensive Properties Overview Examples Expii

Intensive And Extensive Properties YouTube

Extensive And Intensive Properties Thermodynamics Physical
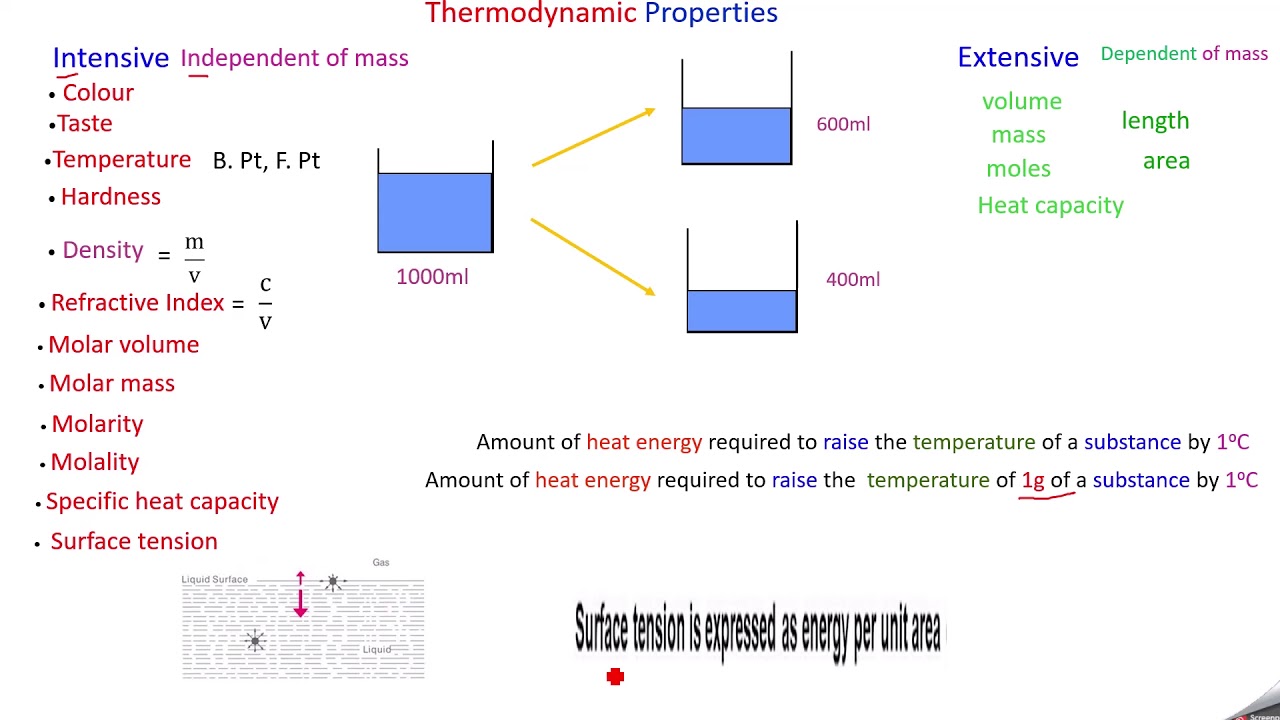
Thermodynamic Properties Intensive And Extensive YouTube

https://themechanicalengineering.com/thermodynamic-properties
Oct 31 2021 0183 32 It shows that the intensive property has a definite value and it is independent of mass it represents a point function Extensive property The property of the system which depends upon the size and mass

https://en.wikipedia.org/wiki/Intensive_and_extensive_properties
The distinction between intensive and extensive properties has some theoretical uses For example in thermodynamics the state of a simple compressible system is completely specified by two independent intensive properties along
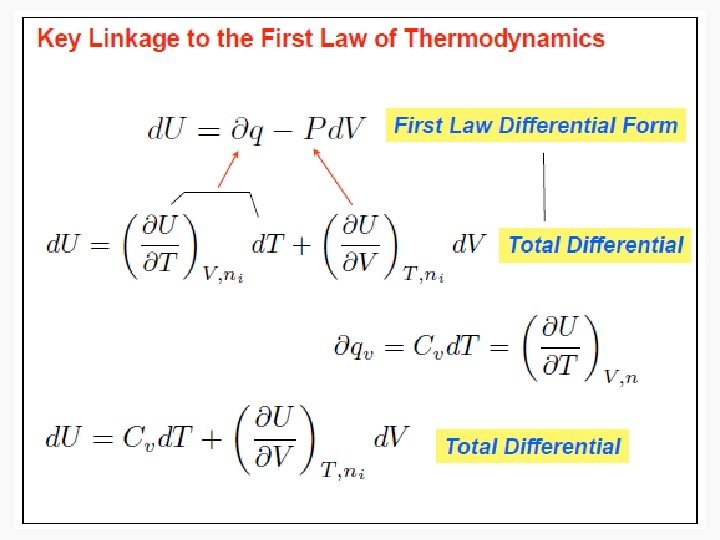
https://eng.libretexts.org/Bookshelves/Mechanical
Jan 29 2024 0183 32 Properties such as mass volume internal energy enthalpy and entropy are extensive properties Their values change accordingly as the mass of a system changes Intensive properties are independent of the mass of a system

https://www.thoughtco.com/intensive-vs-extensive
Dec 4 2019 0183 32 Key Takeaways Intensive vs Extensive Properties The two types of physical properties of matter are intensive properties and extensive properties Intensive properties do not depend on the quantity of matter Examples include density state of matter and temperature Extensive properties do depend on sample size

https://chem.libretexts.org/Bookshelves/Physical
Sep 10 2022 0183 32 Nevertheless a given thermodynamic property of a system can be classified as either intensive or extensive Intensive Properties The magnitude of an intensive variable does NOT depend on the amount of chemical substance in a
MP 1 2 What is the difference between extensive and intensive properties Intensive properties are properties that do not depend on the quantity of matter For example pressure and temperature are intensive properties Energy volume and enthalpy are all extensive properties Their value depends on the mass of the system May 22 2019 0183 32 Thermodynamic properties can be divided into two general classes Extensive properties An extensive property is dependent upon the amount of mass present or upon the size or extent of a system Mass total volume and energy are examples of extensive properties
Jan 15 2024 0183 32 Examples of intensive properties in thermodynamics include temperature pressure density and specific heat capacity Extensive properties in thermodynamics are those that depend on the size or extent of the system Examples of extensive properties in thermodynamics include internal energy enthalpy entropy and volume