What Is Bond Polarity Simple Definition Jan 16 2024 0183 32 What is Polarity Polarity refers to the degree of uneven charge distribution within a molecule It is a consequence of the electronegative differences between atoms involved in
Apr 22 2024 0183 32 Bond polarity can be defined as the difference in electronegativity EN between the two bonding atoms The greater the difference in Mar 13 2018 0183 32 In chemistry polarity refers to the way in which atoms bond with each other When atoms come together in chemical bonding they share
What Is Bond Polarity Simple Definition
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg) What Is Bond Polarity Simple Definition
What Is Bond Polarity Simple Definition
https://www.thoughtco.com/thmb/ROT8XEkPr9HqiBReQNNK6KHN1DQ=/1500x1000/filters:fill(auto,1)/PolarConvalentBond-58a715be3df78c345b77b57d.jpg
Bond polarity refers to the distribution of electric charge across a chemical bond between two atoms If the bond is non polar the charge is evenly distributed
Pre-crafted templates offer a time-saving option for creating a diverse variety of files and files. These pre-designed formats and layouts can be utilized for different personal and professional projects, including resumes, invites, flyers, newsletters, reports, discussions, and more, improving the material development procedure.
What Is Bond Polarity Simple Definition
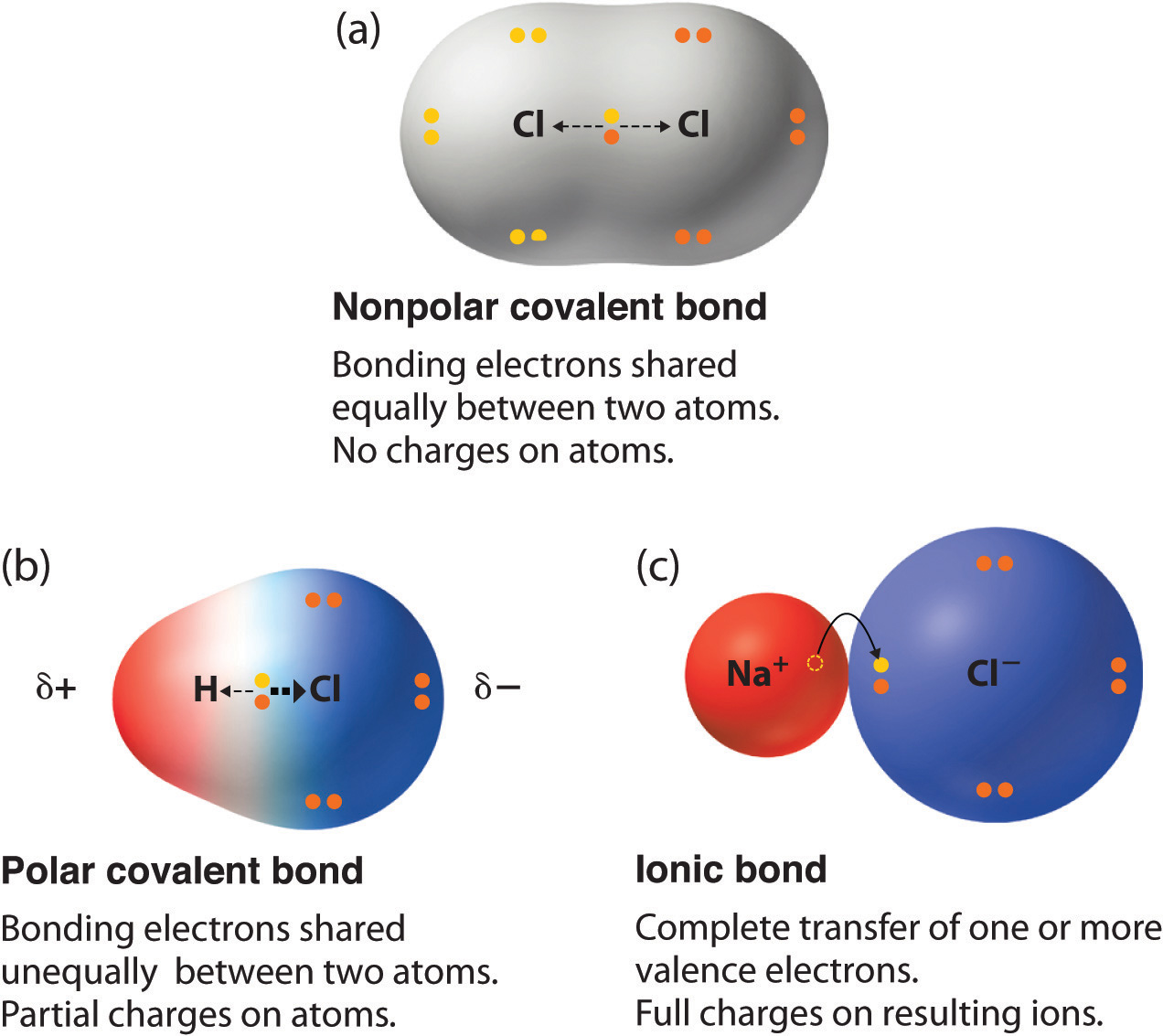
Polar Covalent Bonds
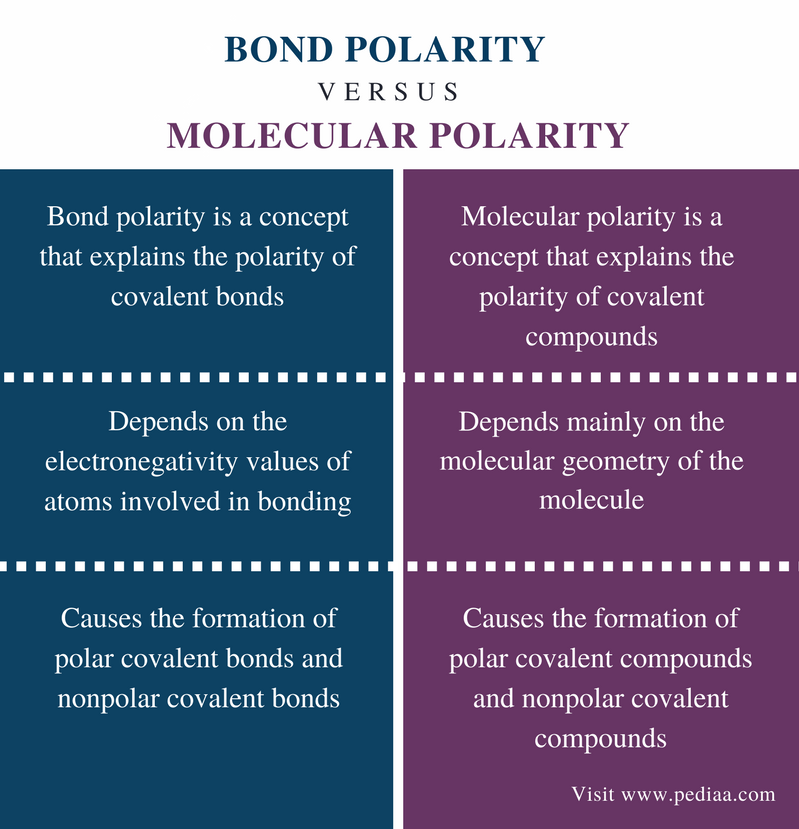
Difference Between Bond Polarity And Molecular Polarity Definition
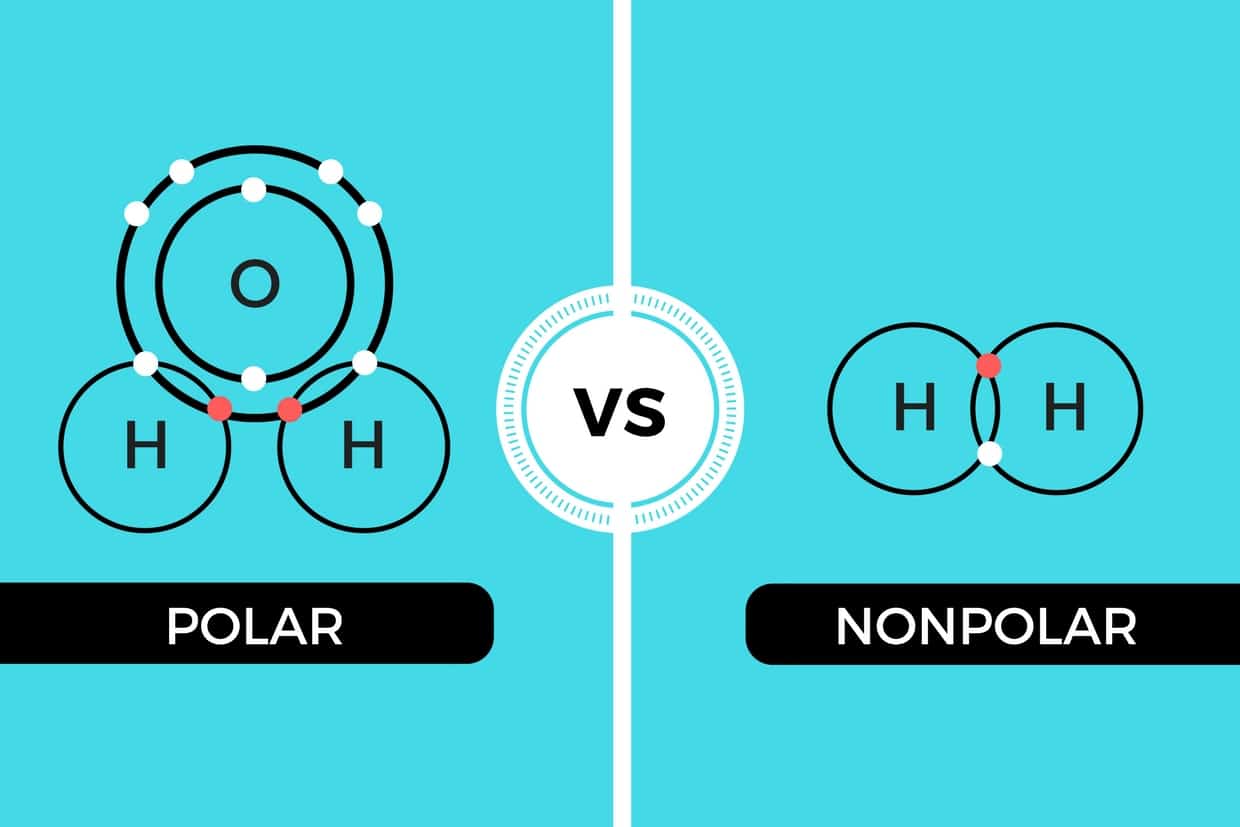
Non Polar Molecules Examples Slideshare
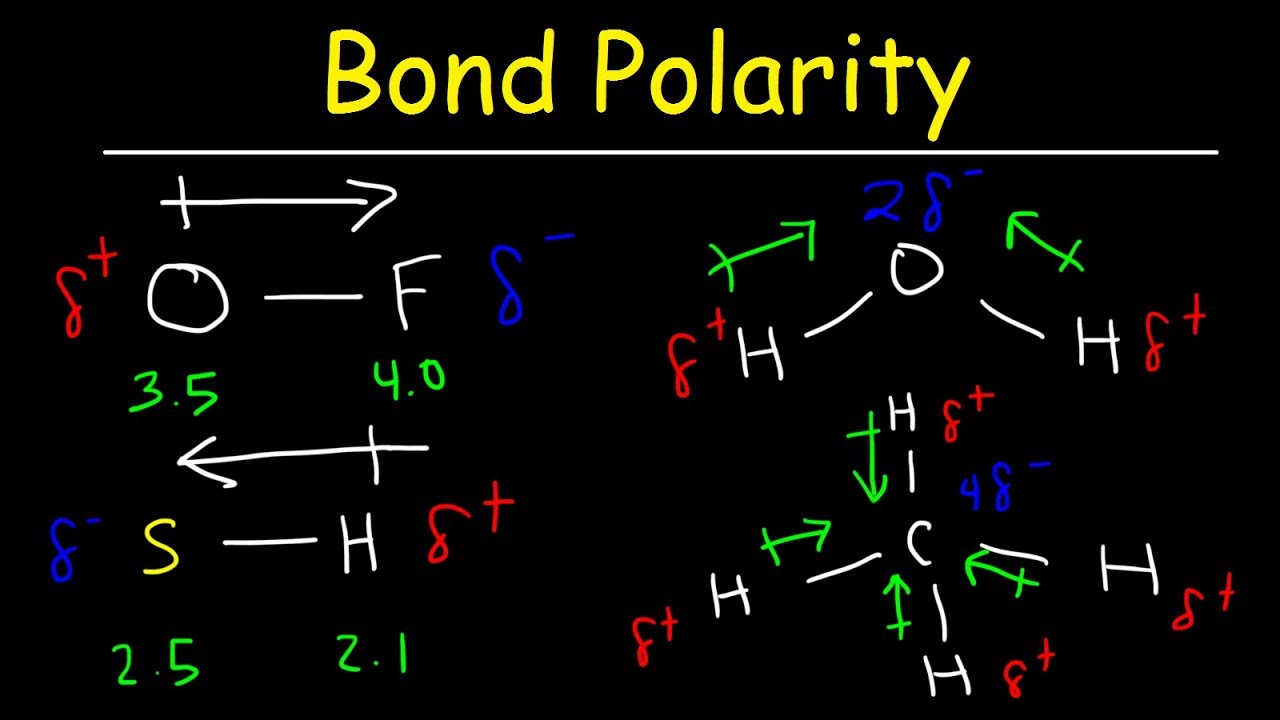
Bond Polarity Electronegativity And Dipole Moment Chemistry Practice
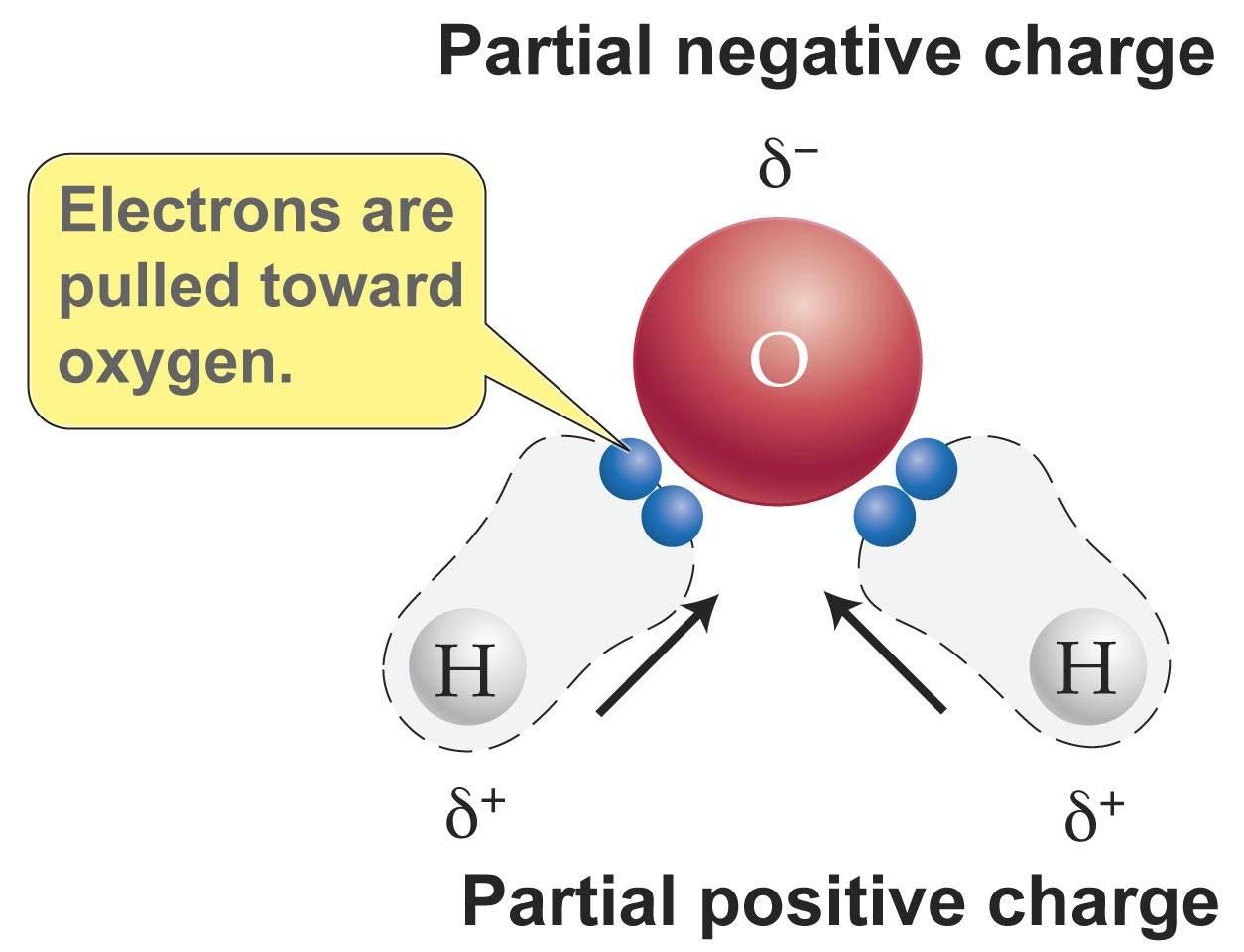
Water Lewis Structure Polarity
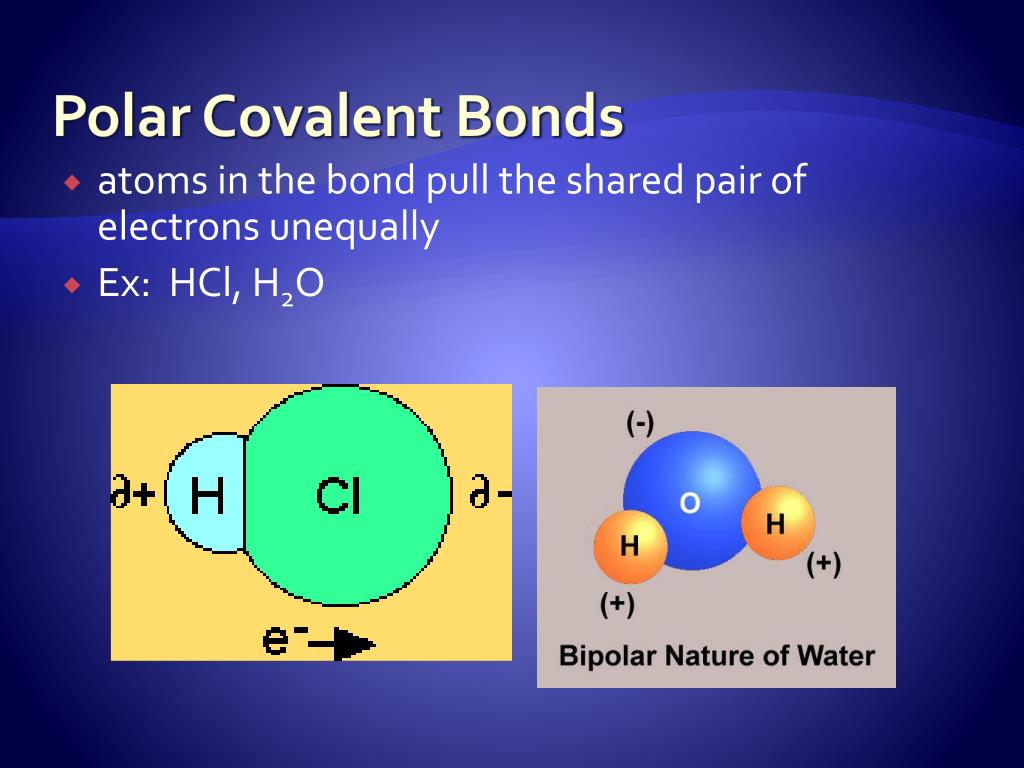
Polar Covalent Bond Mga Katangian At Halimbawa Agham 2022 Mobile Legends
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg?w=186)
https://www.chemistrylearner.com › polarity
Polarity refers to the condition in which the electric charges on a molecule are separated leading to a partial positive charge at one end and a partial

https://chem.libretexts.org › Bookshelves
This page explains bond polarity in chemistry detailing how electronegativity affects electron sharing or transfer between atoms It describes that large

https://byjus.com › chemistry › polarity
What does polarity mean in chemistry The distribution of electrical charge over the atoms connected by the bond is referred to as polarity in chemical bonding
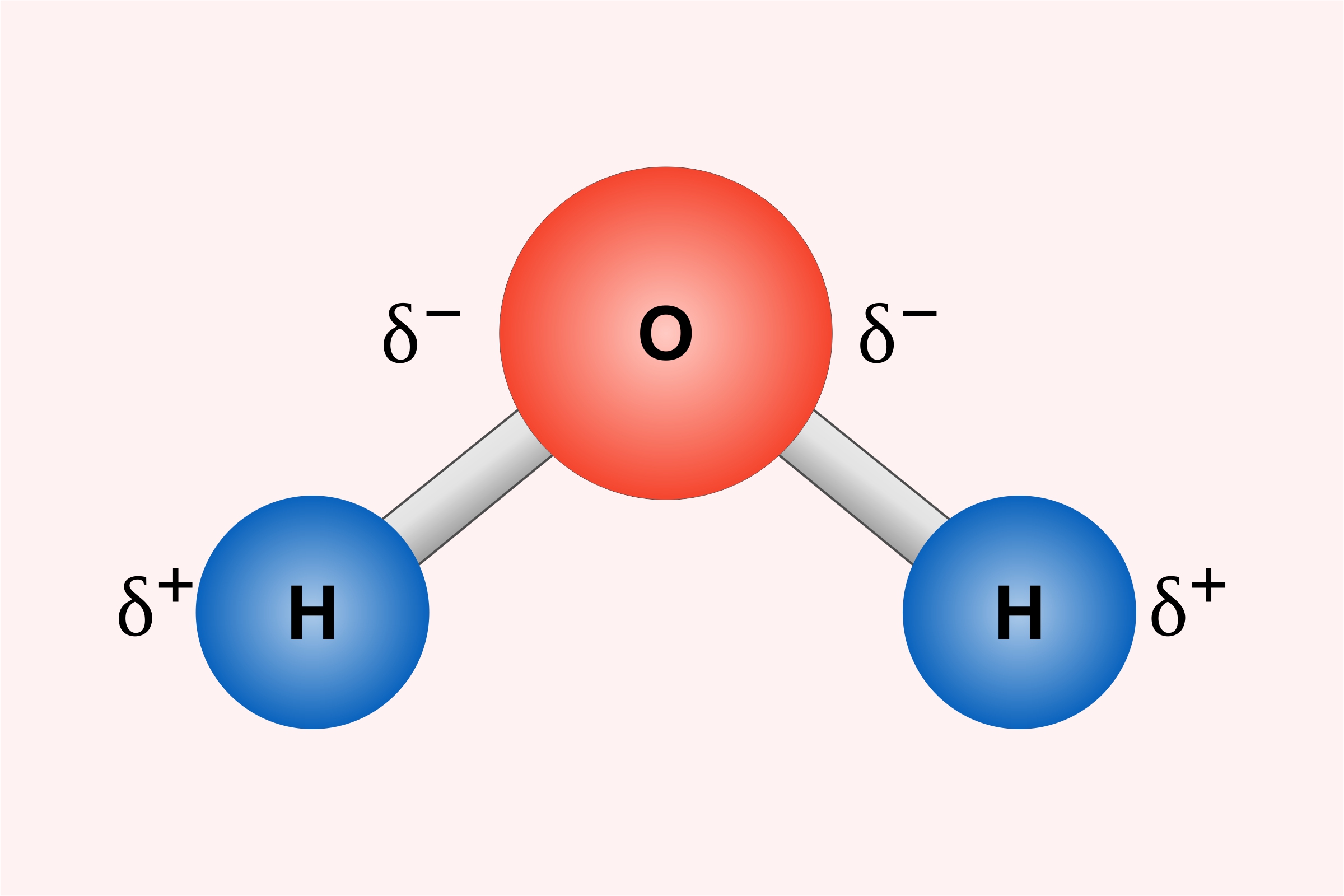
https://www.britannica.com › science › pol…
Polarity in chemical bonding the distribution of electrical charge over the atoms joined by the bond While bonds between identical atoms such as two of

https://www.clrn.org › what-is-bond-polarity-in-chemistry
Dec 27 2024 0183 32 Bond polarity is a fundamental concept in chemistry describing the unequal sharing of electrons between two atoms in a covalent bond By grasping the principles of
Jul 21 2024 0183 32 A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed This causes the molecule to have a slight electrical dipole Apr 22 2025 0183 32 In chemistry polarity describes how electrons are shared between atoms in a chemical bond When atoms with different electronegativities bond together one atom pulls the
Bond polarity refers to the unequal sharing of electrons between atoms in a covalent bond resulting in an uneven distribution of electron density and the formation of partial positive and