Writing Formulas For Ionic Compounds With Transition Metals Worksheet WEB Oct 25 2022 0183 32 Naming ionic compounds with transition metals Liveworksheets transforms your traditional printable worksheets into self correcting interactive exercises that the students can do online and send to the teacher
WEB This is a follow up to the ionic nomenclature worksheet that introduces transition metals Students will learn how to assign Roman numerals to transition metals to indicate their charge and how to use anion charges to figure out WEB Naming Ionic Compounds with Transition Metals Write the name of transition metal as shown on the Periodic Table Write the name and charge for the non metal If you have a polyatomic ion use the Common Ion Table to find and write the formula and charge
Writing Formulas For Ionic Compounds With Transition Metals Worksheet
 Writing Formulas For Ionic Compounds With Transition Metals Worksheet
Writing Formulas For Ionic Compounds With Transition Metals Worksheet
https://s3.studylib.net/store/data/009291540_1-c9896bcec5ca814c3605073966d45410.png
WEB Naming Ionic and Covalent Compounds Acids and Hydrates 9 worksheets to practice naming and writing formulas for ionic and covalent compounds including acids and hydrates Each worksheet has a full preview available
Pre-crafted templates offer a time-saving service for creating a varied variety of documents and files. These pre-designed formats and designs can be utilized for different individual and expert projects, consisting of resumes, invitations, leaflets, newsletters, reports, discussions, and more, improving the material creation procedure.
Writing Formulas For Ionic Compounds With Transition Metals Worksheet
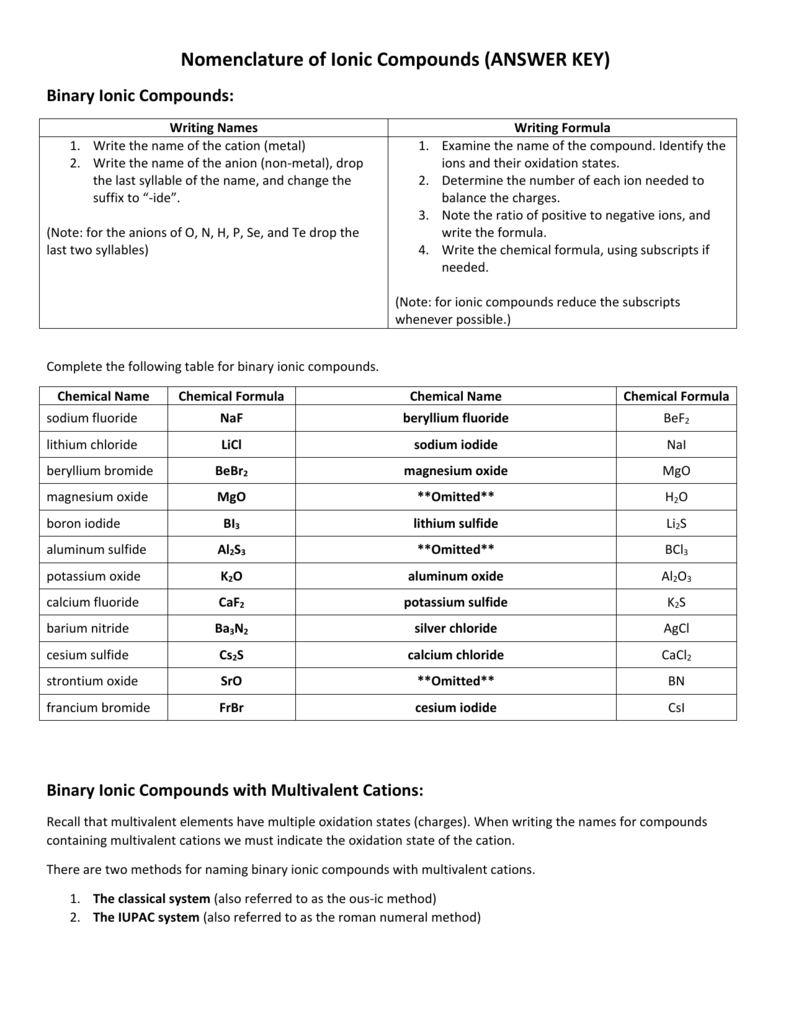
Nomenclature Of Ionic Compounds ANSWER KEY

Ionic Compounds And Metals Worksheet Answers

Naming Chemical Compounds Worksheet Answers
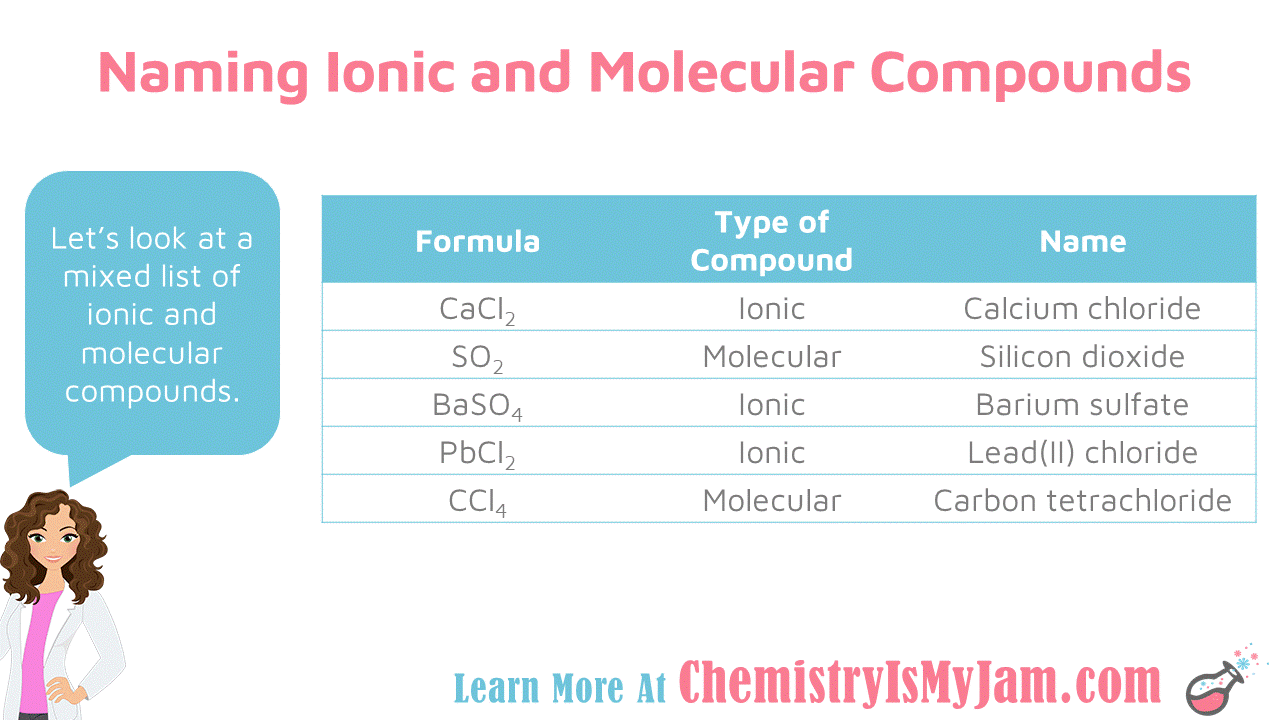
Chemical Bonding Chemistry Is My Jam
.PNG)
Naming Ionic Compounds
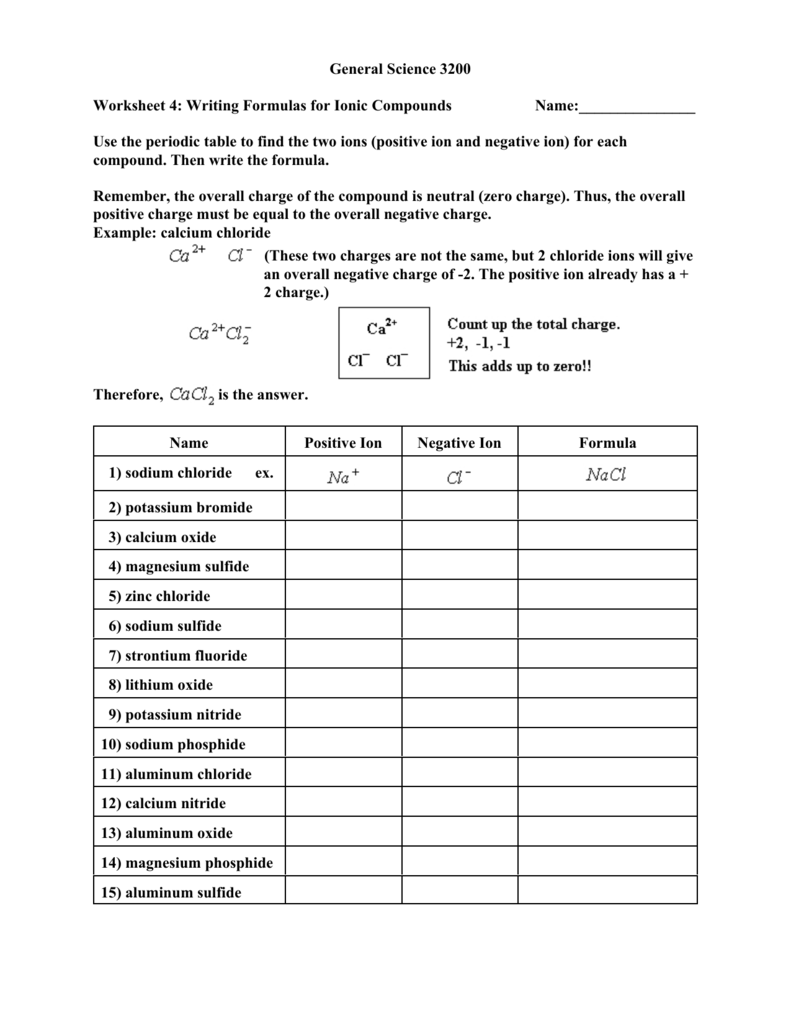
General Science 3200 Worksheet 4 Writing Formulas For Ionic

https://www.sfponline.org/Uploads/71/Naming
WEB Write the formulas for the following ionic compounds 21 lithium acetate 22 iron II phosphate

https://learnwithdrscott.com/wp-content/uploads/
WEB This worksheet presents a widely used system of nomenclature for ionic compounds There are two types of metal cations with different naming conventions discussed separately Part A Fixed charge single charge cations Part B Variable charge multiple charge cations Cations with a single fixed charge Cations have a positive charge

http://www.simplychemistry.org/chemistry/Pearson Chemistry
WEB Binary Ionic Compounds 1 Traditionally common names were based on some of a compound or its 2 What is the general name for compounds composed of two elements They are 3 When writing the formula for any ionic compound the charges of the ions must 4 What are two methods for writing a balanced formula
.PNG?w=186)
https://www.foresthillshs.org/ourpages/auto/2019/3
WEB Mar 7 2019 0183 32 Directions First quickly scan the worksheet and circle any metals as a symbol or as a name that is a transition metal Then either give the same or chemical formula as necessary for each problem below

https://laney.edu/pinar-alscher/wp-content/uploads/
WEB ionic compounds Ionic compounds are written to show the smallest whole number ratio of each ion in the formula The charge is balanced so that the positive charge equals the negative charge For example if I want to build a formula for a compound that contains Mg2 and F I would need one Mg2 and two F I would write the formula as MgF
WEB Nomenclature Worksheet 3 Naming of Transition metal salts Like all metals when transition metals combine with a nonmetal they form ionic compounds They differ from the Group A elements in that they can form cations with different charges For example iron can form both Fe2 and Fe3 ions Because of this the charge or oxidation state of WEB For each combination of ions fill in the chart below 22 3 3 0 23 4 0 24 4 2 0 25 3 2 0 26 2 3 0 cation symbol anion symbol cations needed to make a neutral compound
WEB 2 Write formulas for the compounds below Includes molecular and ionic compounds polyatomic ions acids and transition metals Note Not all instructors cover the same ions and some instructors skip transition metal compounds a magnesium bromide b sodium nitrite c calcium nitride d lithium sulfate