Atomic Weight Practice Worksheet Answers Atomic Mass Practice Problems 37 problems 1 PRACTICE PROBLEM How does an element s atomic mass differ from its atomic number 2 PRACTICE PROBLEM Initially the atomic
Calculate the average atomic mass of lithium which occurs as two isotopes that have the following atomic masses and abundances in nature 6 017 u 7 30 and 7 018 u 92 70 Calculate the average atomic mass of an element with the follow isotope information 4 35 have a mass of 49 9461 amu 83 79 have amass of 51 9405 amu 9 50 have a mass of 52 9407 amu and 2 36 have a mass of 53 9389
Atomic Weight Practice Worksheet Answers
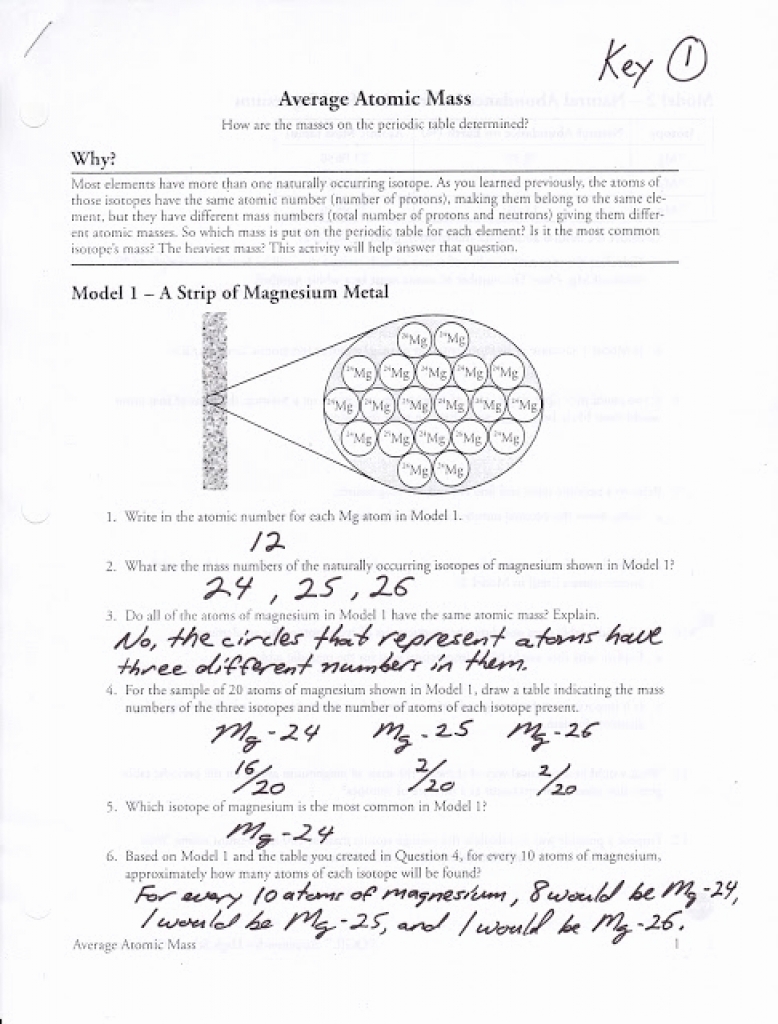 Atomic Weight Practice Worksheet Answers
Atomic Weight Practice Worksheet Answers
https://www.unmisravle.com/wp-content/uploads/2018/03/average_atomic_mass_worksheet_answer_key_1.jpg
Calculate the elemental atomic mass of Mg if the naturally occurring isotopes are 24 Mg 25 Mg and 26 Mg Their masses and abundances are as follows
Pre-crafted templates offer a time-saving service for developing a varied series of documents and files. These pre-designed formats and designs can be used for numerous personal and expert jobs, consisting of resumes, invitations, leaflets, newsletters, reports, presentations, and more, streamlining the material creation process.
Atomic Weight Practice Worksheet Answers
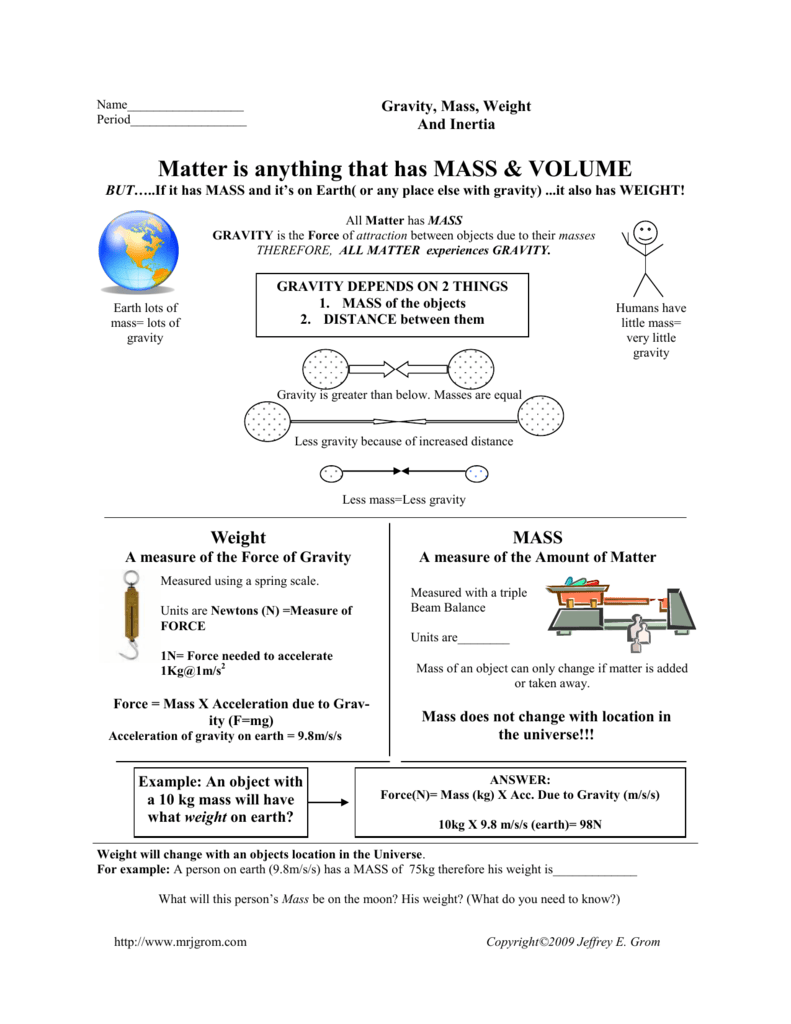
Mass And Weight Worksheet Copyright 2009 Jeffrey E Grom
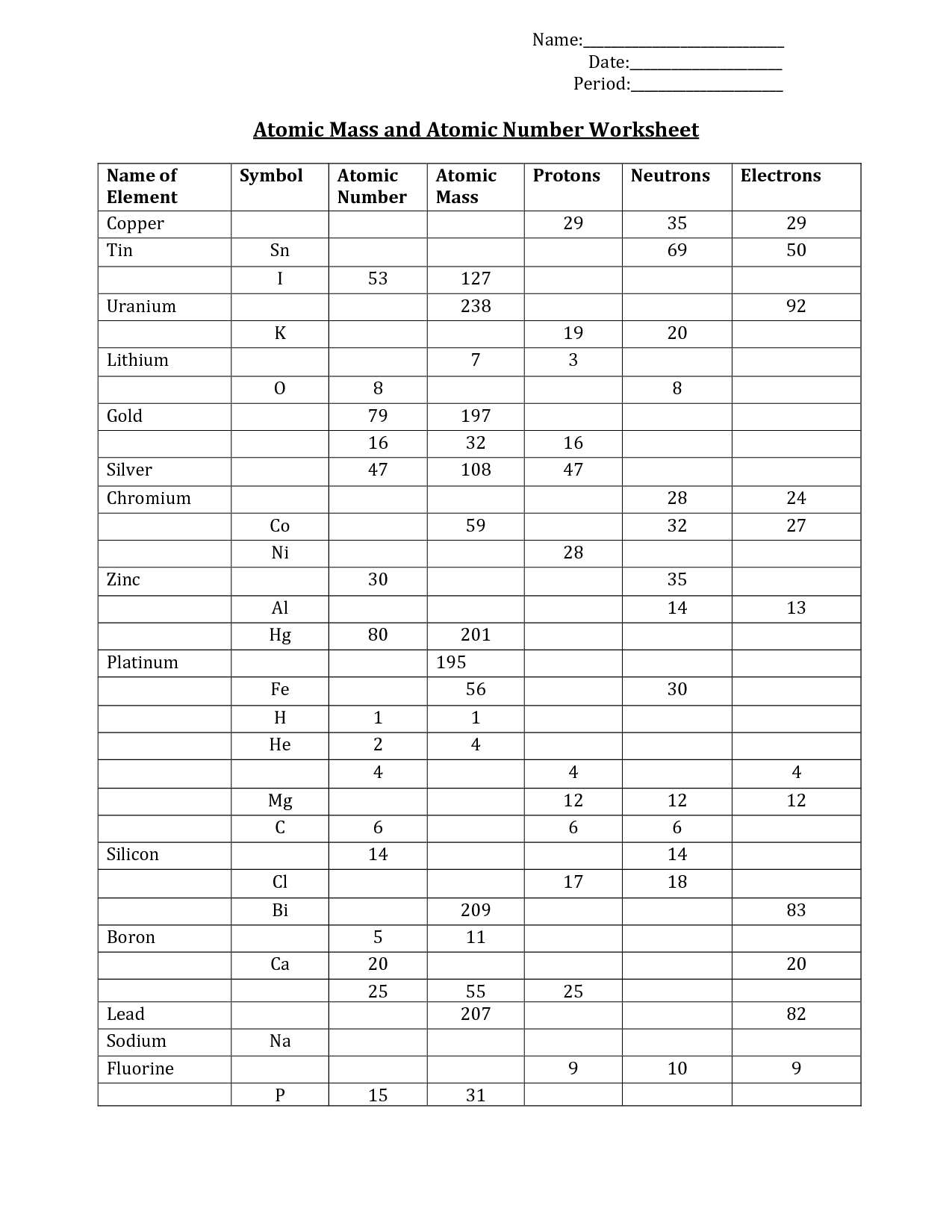
16 Atomic Structure Practice Worksheet Worksheeto
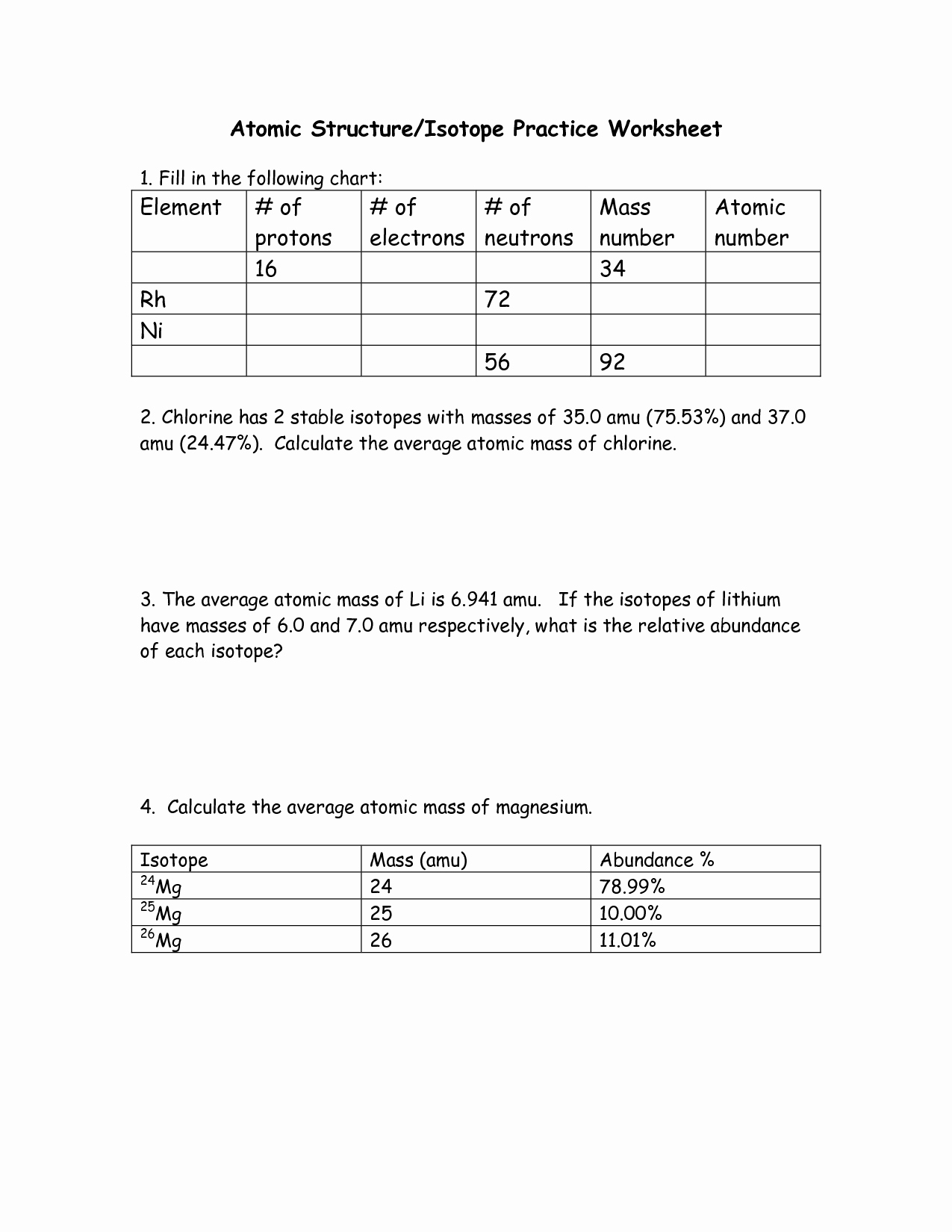
50 Average Atomic Mass Worksheet Answers Chessmuseum Template Library
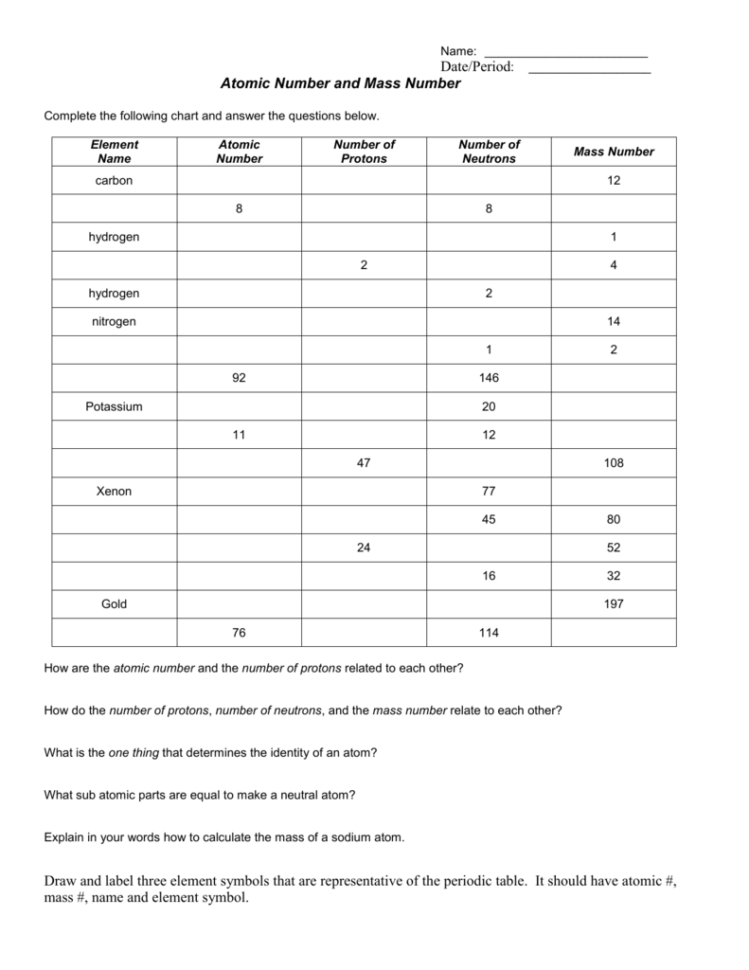
Atomic Number And Mass Number Worksheet Db excel
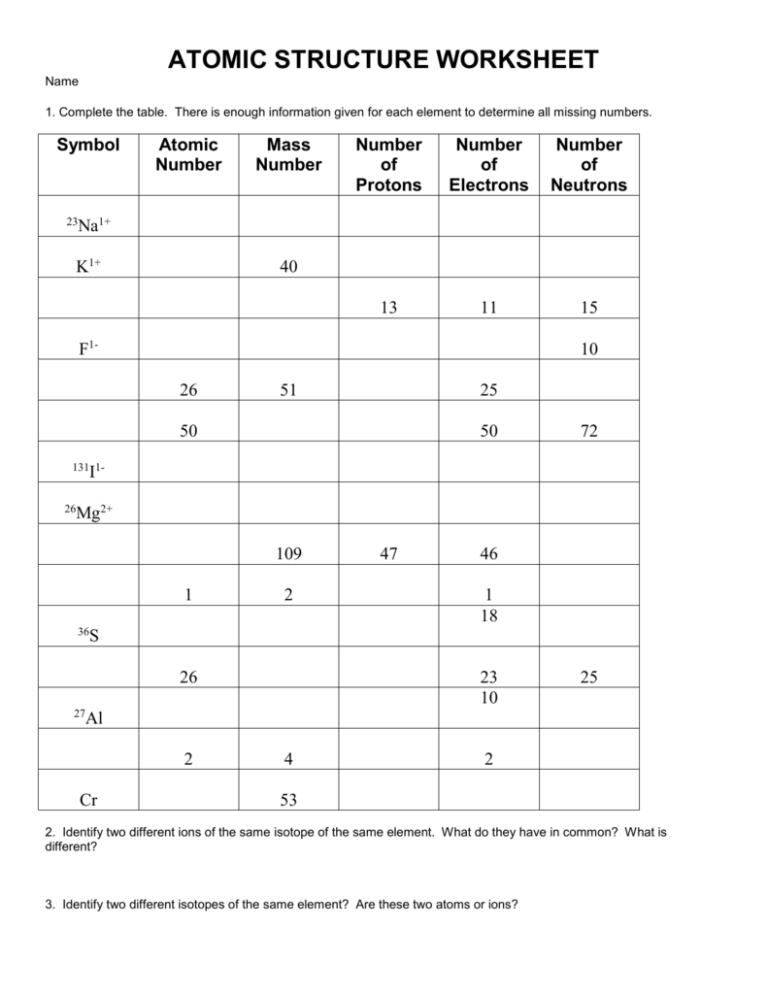
ATOMIC STRUCTURE WORKSHEET
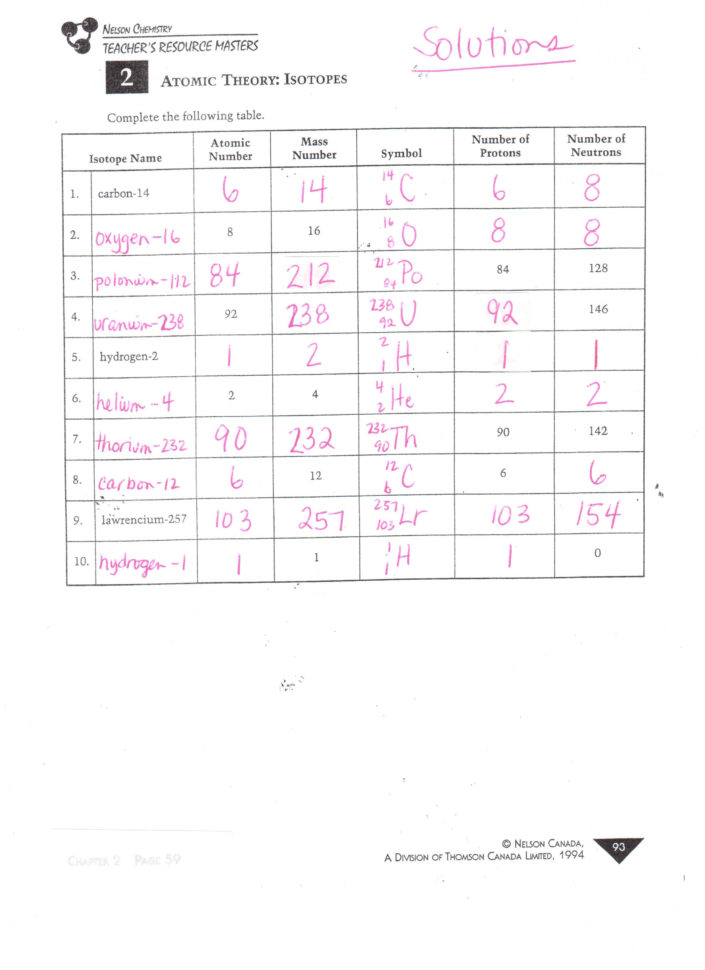
Atomic Structure Practice Worksheet Db excel

https://siprogram.weebly.com/uploads/1/2/0/5/
Isotopic abundance practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an

http://mrsochoachemistry.weebly.com/uploads/5/5/atomi…
Calculate the average atomic mass for each element based on the natural abundance of its isotopes Find the average atomic mass for Li if 7 5 of Li atoms are 6Li with a mass of
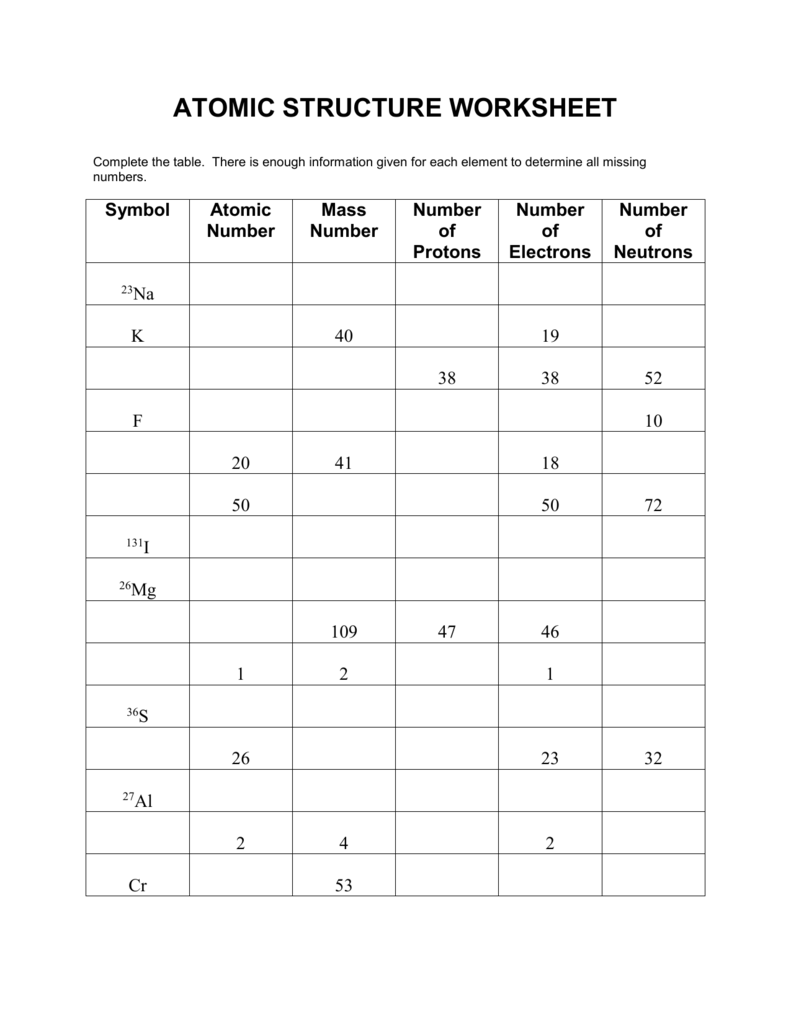
https://atischemistry.weebly.com/uploads/5/4/8/6/
Isotope Practice Worksheet Name Answer Key Class Gr 11 1 Here are three isotopes of an element 12C 6 13C 14C 6 6 a The element is Carbon b The number 6 refers to the Atomic
https://www.hasdk12.org/cms/lib3/PA01001366
1 Four isotopes of lead include lead 204 lead 206 lead 207 and lead 208 The average atomic mass of a lead atom is 207 2 amu Which isotope of lead is likely to be the most abundant 2

https://www.scisheets.co.uk/wp-content/uploads/
RELATIVE ATOMIC MASS The relative atomic mass Ar of atoms is the average mass of all the different isotopes of an element taking into account the amount of each isotope on a scale
May 28 2020 0183 32 Answer Formic acid Its formula has twice as many oxygen atoms as the other two compounds one each Therefore 0 60 mol of formic acid would be equivalent to 1 20 mol May 28 2020 0183 32 An element has the following natural abundances and isotopic masses 90 92 abundance with 19 99 amu 0 26 abundance with 20 99 amu and 8 82 abundance with
Calculate the average atomic mass of bromine One isotope of bromine has an atomic mass of 78 92amu and a relative abundance of 50 69 The other major isotope of bromine has an