Atoms Isotopes And Ions Worksheet Answer Key Pdf WEB For each of the following isotopes write the of protons neutrons and electrons Fill in the isotope names and any missing information including isotope numbers from the chart Use your periodic table and the information provided
WEB Anions Have a negative charge Have gained electrons Ion symbol To write the ion symbol you must write the element symbol with the charge written on the top right Example Ca2 Zn2 Ag1 Atoms and Ions Worksheet Fill in WEB 1 If Li loses an electron to another atom why does it have a have a 1 charge 2 If N gains 3 electrons from other atoms why does it have a 3 charge 3 a What do you think happens to atomic radius size of a cation amp why b an anion amp why
Atoms Isotopes And Ions Worksheet Answer Key Pdf
 Atoms Isotopes And Ions Worksheet Answer Key Pdf
Atoms Isotopes And Ions Worksheet Answer Key Pdf
https://i.pinimg.com/originals/c6/4e/22/c64e22ccc0996938679029d3a24495fb.jpg
WEB What is an isotope Isotopes are versions of the same element They have the same number of protons and electrons as the element but different mass numbers and number of neutrons What does the number next to isotopes signify The number indicates the isotope s mass number How can you tell isotopes of the same element apart
Pre-crafted templates offer a time-saving solution for producing a varied series of documents and files. These pre-designed formats and designs can be made use of for various individual and expert jobs, including resumes, invitations, leaflets, newsletters, reports, discussions, and more, enhancing the content creation process.
Atoms Isotopes And Ions Worksheet Answer Key Pdf

Chemistry Worksheet Isotope Notation

Isotopes Ions And Atoms Worksheet 2 Answer Key
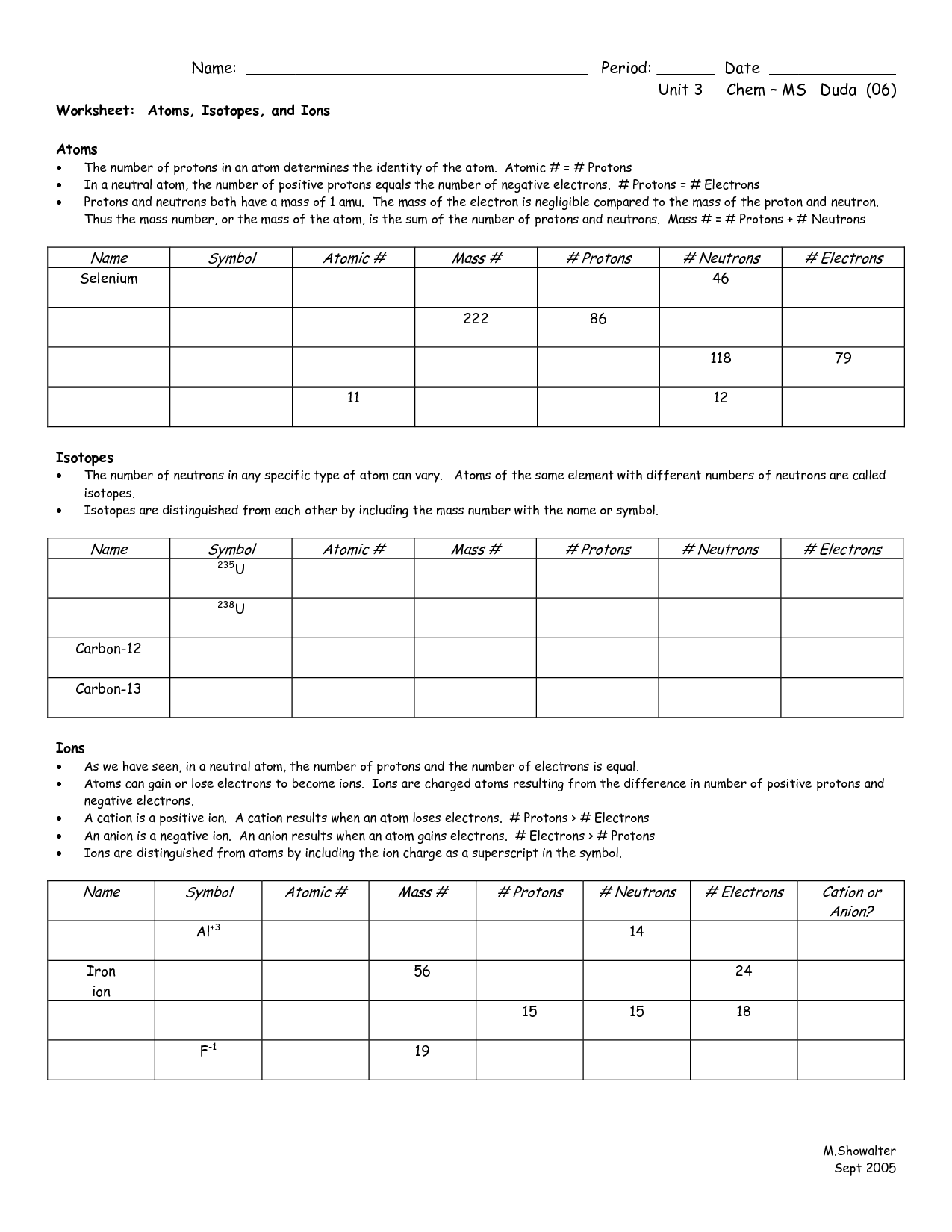
17 Best Images Of Which Atom Is Which Worksheet Drawing Atoms
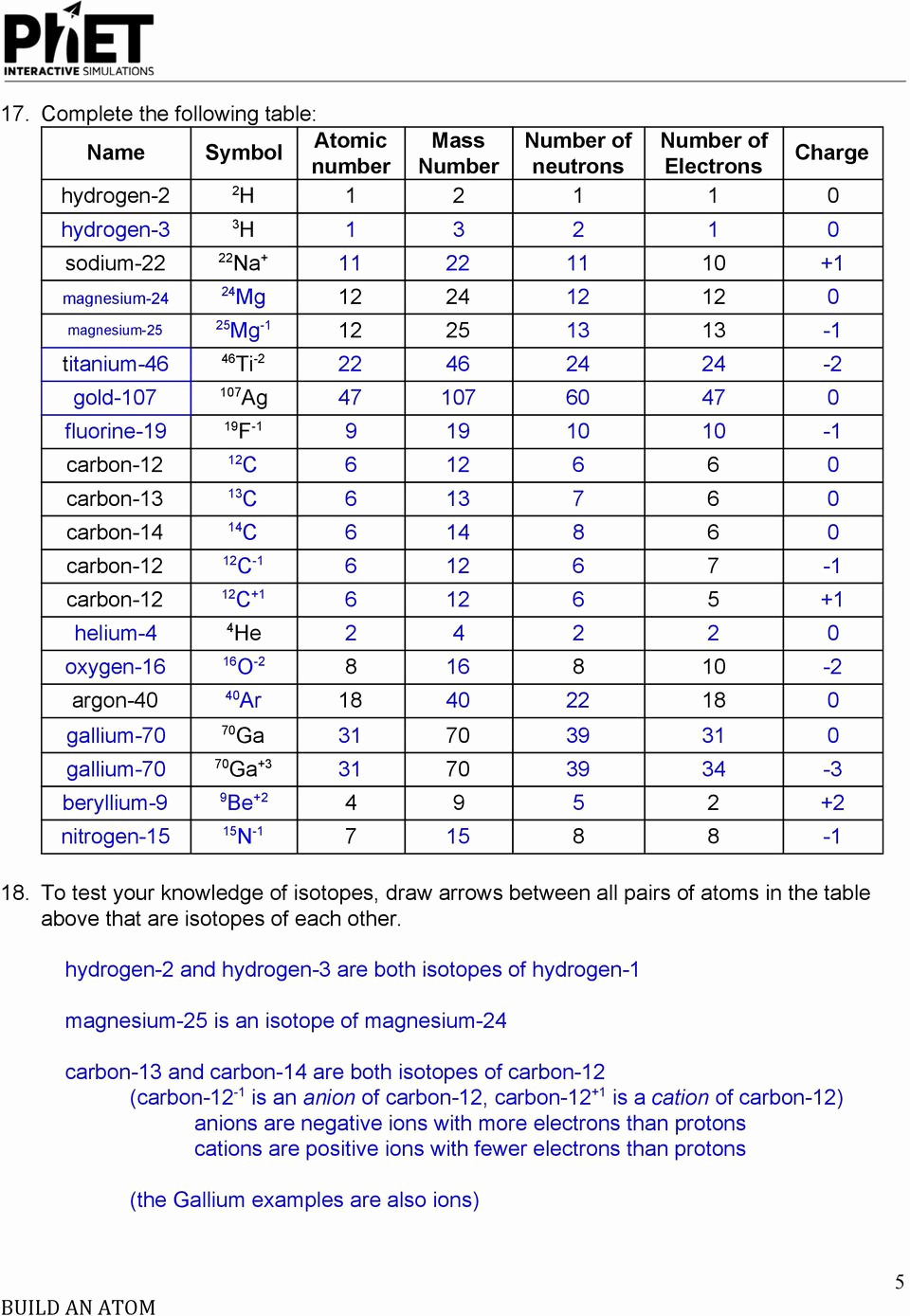
Isotopes And Ions Worksheet Answer Key Long Division Db excel
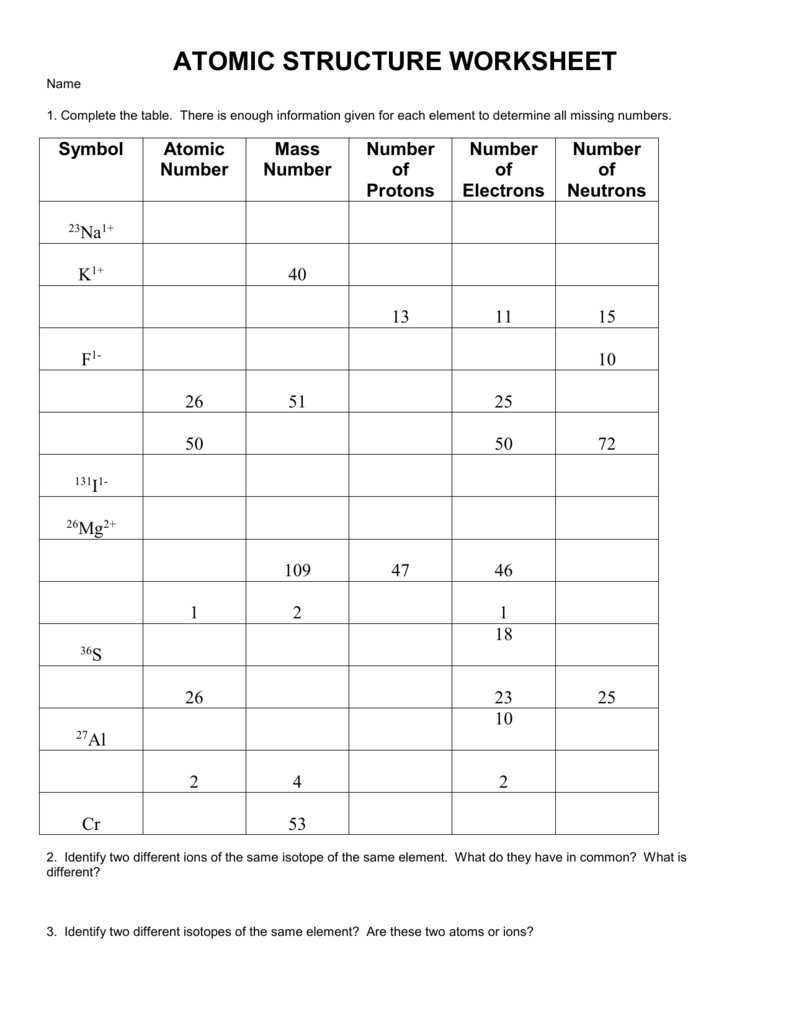
Ions And Isotopes Worksheet
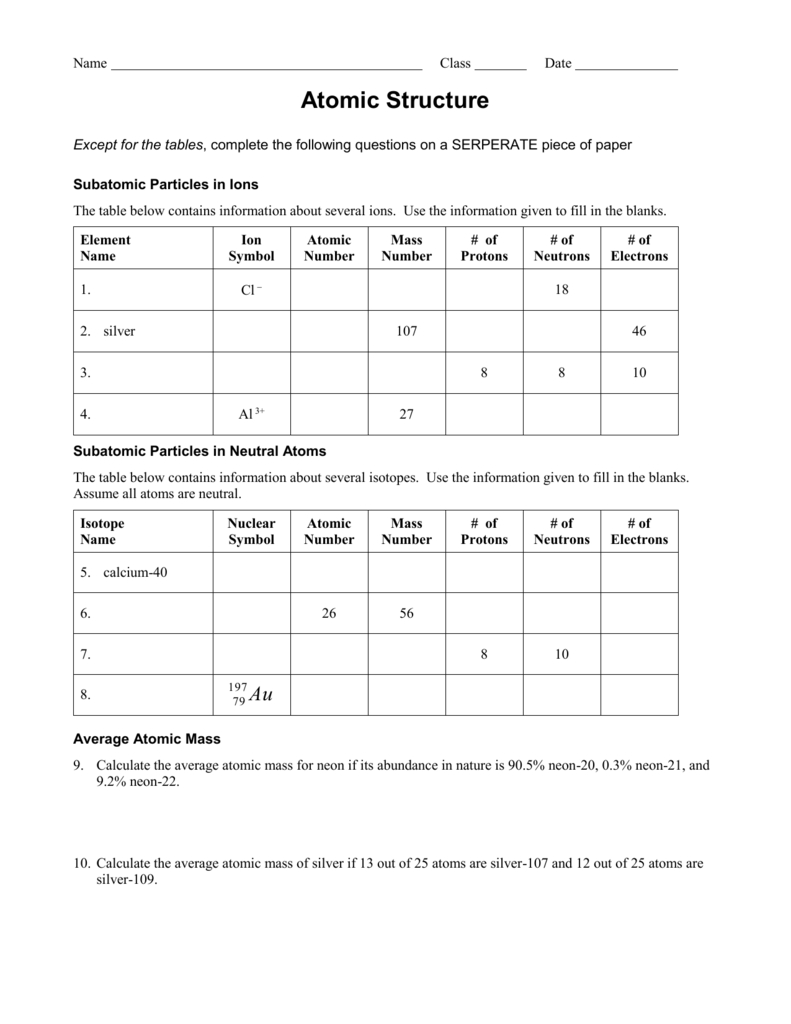
Atomic Structure Worksheet With Answers Islero Guide Answer For

https://higginschemistry.weebly.com//ions_-_key.pdf
WEB Ions How are ions made from neutral atoms Why You have learned that not all atoms of an element are the same Variation in the number of neutrons results in different isotopes of the element In this activity we will explore another variation that can take place the loss and gain of electrons

https://mrscowley.weebly.com/uploads/2/8/3/7/
WEB 1 What is an ion 2 What does the number next to the ions signify Complete the following table using the periodic table in the back of your book 16 62 Wh o 3 How can you tell isotopes apart Germanium 64 For each of the following isotopes write the number of protons neutrons and electrons

https://www.sfponline.org/Uploads/382/Isotopes Review.pdf
WEB Atoms of the same element with different numbers of neutrons are called isotopes Isotopes are distinguished from each other by including the mass number with the name or symbol

https://scilearn.sydney.edu.au//chem1001/ws2.pdf
WEB Model 2 Isotopes Atoms of the same element have the same number of protons but not necessarily the same number of neutrons These are called isotopes This is why on the periodic table the mass numbers are not integers they are an average of all the different isotopes of a given element Different isotopes have different

https://www.stocktonusd.net/cms/lib/CA01902791
WEB 1 What is an isotope 2 What does the number next to isotopes signify 3 How can you tell isotopes apart For each of the following isotopes write the number of protons neutrons and electrons Fill in the isotope names and any missing information including isotope numbers from the chart Use your periodic table and the information provided
WEB Aug 15 2020 0183 32 3 Predict the charge of the most stable ion of each atom 4 Predict the chemical formula and name for the ionic compound formed by the following 5 Predict if the following compounds are ionic or molecular then name appropriately WEB The document provides the number of protons neutrons and electrons for various isotopes listed An isotope is a version of an element that has the same number of protons and electrons but a different number of neutrons giving it a different mass number
WEB This document defines atoms isotopes and ions Atoms are identified by their number of protons Isotopes are atoms of the same element with different numbers of neutrons Ions are formed when atoms gain or lose electrons becoming positively or negatively charged