Covalent Bonding Worksheet Answer Key Pdf Chapters 6 and 7 Practice Worksheet Covalent Bonds and Molecular Structure How are ionic bonds and covalent bonds different Describe the relationship between the length of a bond and the strength of that bond Identify the type s of bond s found in the following molecules CCl4
1 How are ionic bonds and covalent bonds different Ionic bonds result from the transfer of electrons from one atom to another Covalent bonds result from two atoms sharing electrons 2 Describe the relationship between the length of a Mar 13 2023 0183 32 Learning Objectives Be able to define covalent bonds polar covalent bonds ionic bonds electronegativity dipoles formal charge molecular formula structural formula and electron dot formula Be able to recognize whether the type of bond between two atoms is covalent polar covalent or ionic
Covalent Bonding Worksheet Answer Key Pdf
 Covalent Bonding Worksheet Answer Key Pdf
Covalent Bonding Worksheet Answer Key Pdf
https://img.yumpu.com/50805616/1/500x640/covalent-bonding-worksheet-colina-middle-school.jpg
Jul 19 2022 0183 32 Propose a bonding scheme that gives each atom the correct number of covalent bonds Hint the two carbon atoms are in the center of a linear molecule How many carbon carbon single bonds linked together are needed to make a carbon chain that is 1 000 cm long
Templates are pre-designed files or files that can be used for different functions. They can save effort and time by providing a ready-made format and design for creating various type of material. Templates can be used for individual or expert jobs, such as resumes, invitations, leaflets, newsletters, reports, discussions, and more.
Covalent Bonding Worksheet Answer Key Pdf
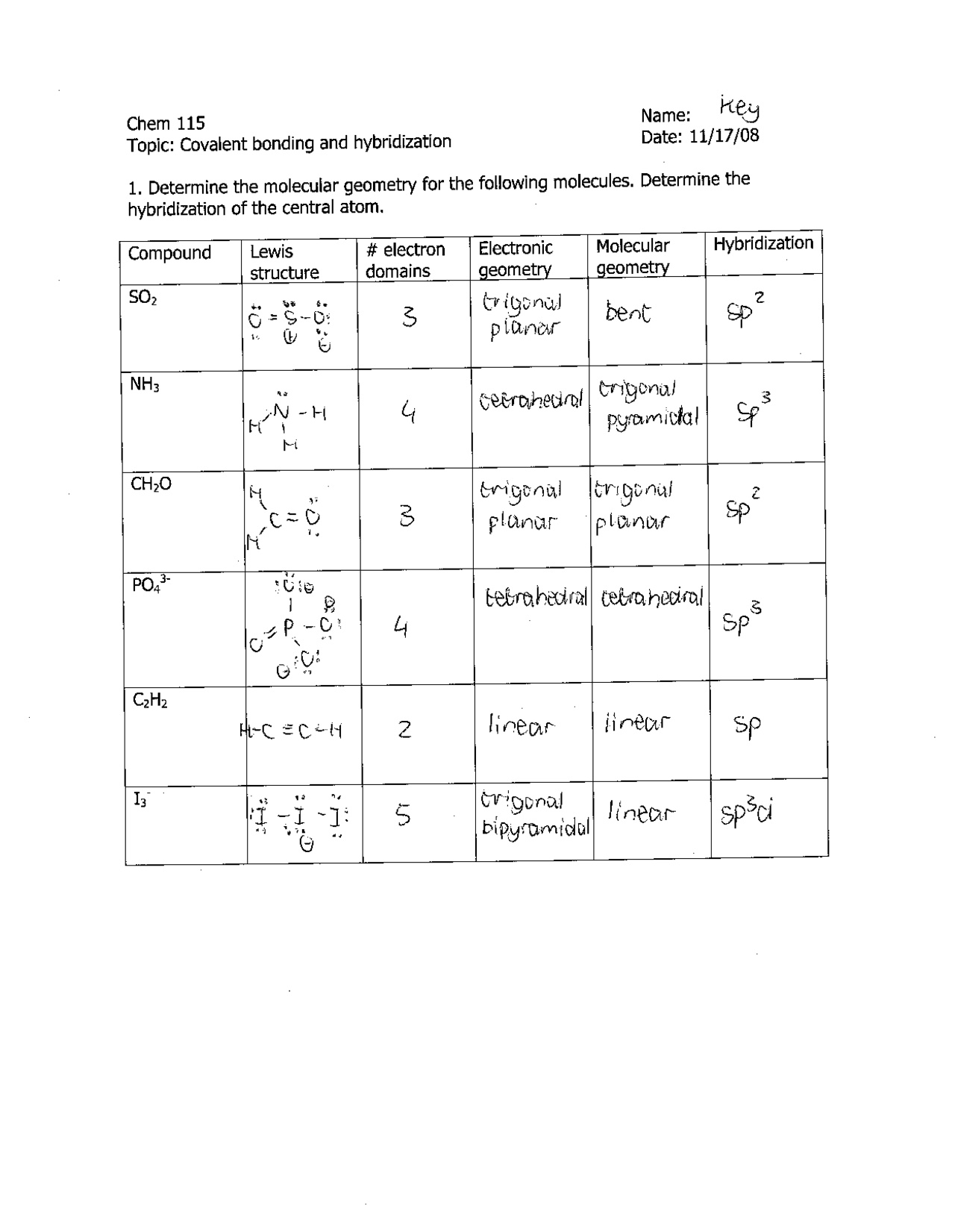
Covalent Bonding and Hybridization - Key Answers | CHEM 115 | Assignments Chemistry | Docsity
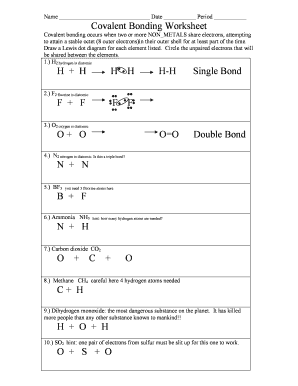
Covalent Bonding Worksheet Answers - Fill Online, Printable, Fillable, Blank | pdfFiller

Chemical Bonding-Answers | PDF | Ionic Bonding | Ion
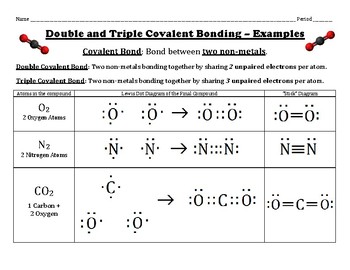
Double and Triple Covalent Bonding Using Lewis Dot Structures by Chemistry Wiz

Covalent Bonding Worksheet Answers - Fill and Sign Printable Template Online

SOLUTION: Ionic Bonding Lewis Dot Structure & Covalent Bonding Worksheet - Studypool

http://www.simplychemistry.org/chemistry/Pearson Chemistry
after reading Lesson 8 2 answer the following questions the octet rule in covalent Bonding 1 What usually happens to the electron configuration of an atom when it forms a covalent Circle the letter of each type of covalent bond that can be formed when p atomic orbitals overlap a pi b beta c sigma d alpha

https://scilearn.sydney.edu.au//chem1001/ws3.pdf
The interaction between two non metals is covalent Two or more non metals form covalent bonds In a covalent bond two atoms share their electrons in order for each to gain a noble gas configuration For most of the atoms you come across this will mean having 8 electrons in the valence shell the octet rule

https://www.saultschools.org/cms/lib/MI17000143
Covalent bonding occurs when two or more NON METALS share electrons attempting to attain a stable octet 8 outer electrons in their outer shell for at least part of the time Draw a Lewis dot diagram for each element listed Circle the unpaired electrons that will be shared between the elements H2 hydrogen is diatomic

https://ch301.cm.utexas.edu/worksheets/Ionic
COVALENT BONDING Name Covalent bonding occurs when two or more nonmetals share electrons attempting to attain a stable octet of electrons at least part of the time For example Note that hydrogen Is content with 2 not 8 electrons Show how covalent bonding occurs in each of the following pairs of atoms

https://mi01000971.schoolwires.net/cms/lib
1 Hydrogen Hydrogen Diatomic Element O 1 Write the symbols for each element step 1 2 Use Fruity Pebbles or other cereal candy with more than one color to create the Lewis structure for each 3 Rearrange the electrons or cereal pieces to pair up electrons from each atom 4 Draw circles to show the sharing of electrons 5 Draw
ChemThink Covalent Bonding ovalent bonding forms when atoms are electrons When two atoms get close enough the nucleus attracts the other atom s Protons Neutrons Before bonding the atom s electrons spend most of their time the nuclei of each atom Once bonded the electrons spend most of their time the two nuclei Atoms must be able Sep 11 2023 0183 32 Be able to define covalent bonds polar covalent bonds ionic bonds electronegativity dipoles molecular formula structural formula and electron dot formula Be able to recognize whether the type of bond between two atoms is
Covalent bonds are formed between atoms when pairs of electrons are shared between atoms A covalent bond is between two nonmetals Electrons are transferred shared so that each atom may reach a more stable electron configuration i e the noble gas configuration which contains 8 valence electrons This is called octet rule Metals amp Non Metals