Covalent Bonding Worksheet Answers Chapter 8 WEB melting a network solid would require breaking covalent bonds throughout the solid These are the vocabulary and key questions for chapter 8 of the chemistry textbook I hope you appreciate it as much as I do since it took me 1 1 2 hours
WEB Main Idea A chemicals bond s character is related to each atom s attraction for the electrons in the bond Electronegativity Polar Covalent Bond Nonpolar Covalent Bond 16 WEB Chemistry 12th Edition answers to Chapter 8 Covalent Bonding 8 2 The Nature of Covalent Bonding 8 2 Lesson Check Page 238 14 including work step by step written by community members like you Textbook Authors Wilbraham ISBN 10 0132525763 ISBN 13 978 0 13252 576 3 Publisher Prentice Hall
Covalent Bonding Worksheet Answers Chapter 8
 Covalent Bonding Worksheet Answers Chapter 8
Covalent Bonding Worksheet Answers Chapter 8
https://sp-uploads.s3.amazonaws.com/uploads/services/1849125/20210603041942_60b8585e14074_rodrigo__kapila___chemistry___bonding___worksheet___answer_keypage2.png
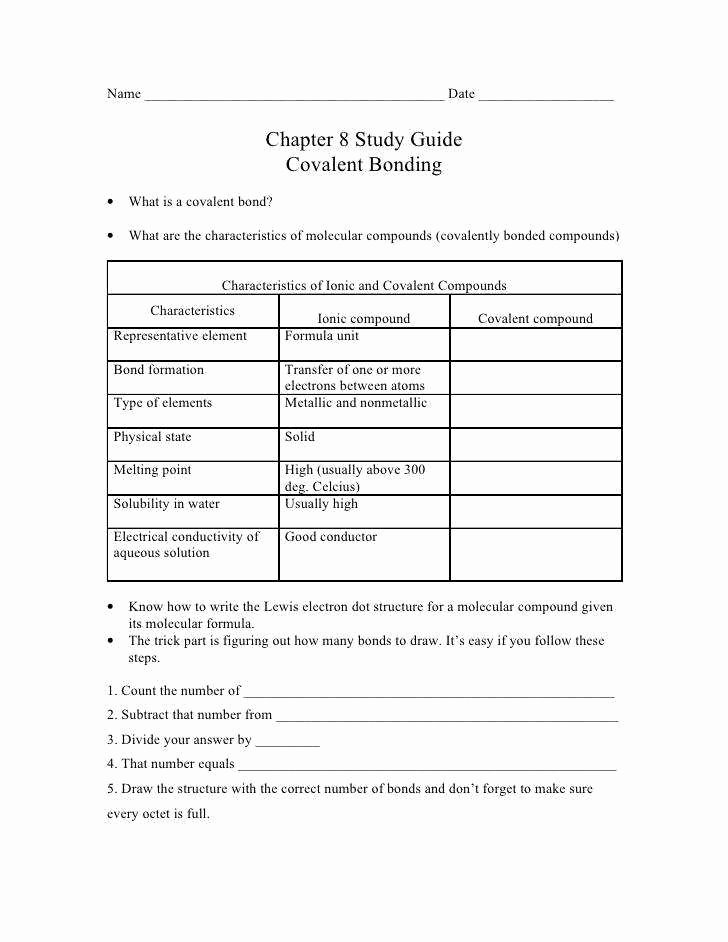
WEB The atoms forming a covalent bond must have relatively equal attraction for the electrons The bonds between the carbon atom and the hydrogen atoms in the compound methane CH 4 are examples of covalent bonds between two different elements
Templates are pre-designed documents or files that can be utilized for numerous functions. They can save time and effort by supplying a ready-made format and layout for developing various sort of content. Templates can be utilized for personal or professional tasks, such as resumes, invitations, flyers, newsletters, reports, discussions, and more.
Covalent Bonding Worksheet Answers Chapter 8

50 Covalent Bonding Worksheet Answers

Chapter 8 Covalent Bonding Worksheet Answers Worksheet

Covalent Bonding Worksheet Answer Key
Covalent Bonding Worksheet Db excel
13 Best Images Of Covalent Formulas Worksheet Writing Ionic Compound
50 Covalent Bonding Worksheet Answers

https://nkscience.weebly.com/uploads/8/7/1/4/
WEB 8 9 10 resonance structure 15 ST 16 NT 21 d True False 13 AT 14 AT Matching 19 b 20 c Part B 11 NT 12 NT Part C 17 e 18 a Section 8 4 Part A Completion 1 equally nonpolar 2 uneq ually 3 polar 4 electronegativities 5 dipole interactions 6 hydrogen bond electronegative 8 oxygen nitrogen or fluorine 9 Section Review 8 1

http://www.schoolisinsession.weebly.com/uploads/1/2/3/5/
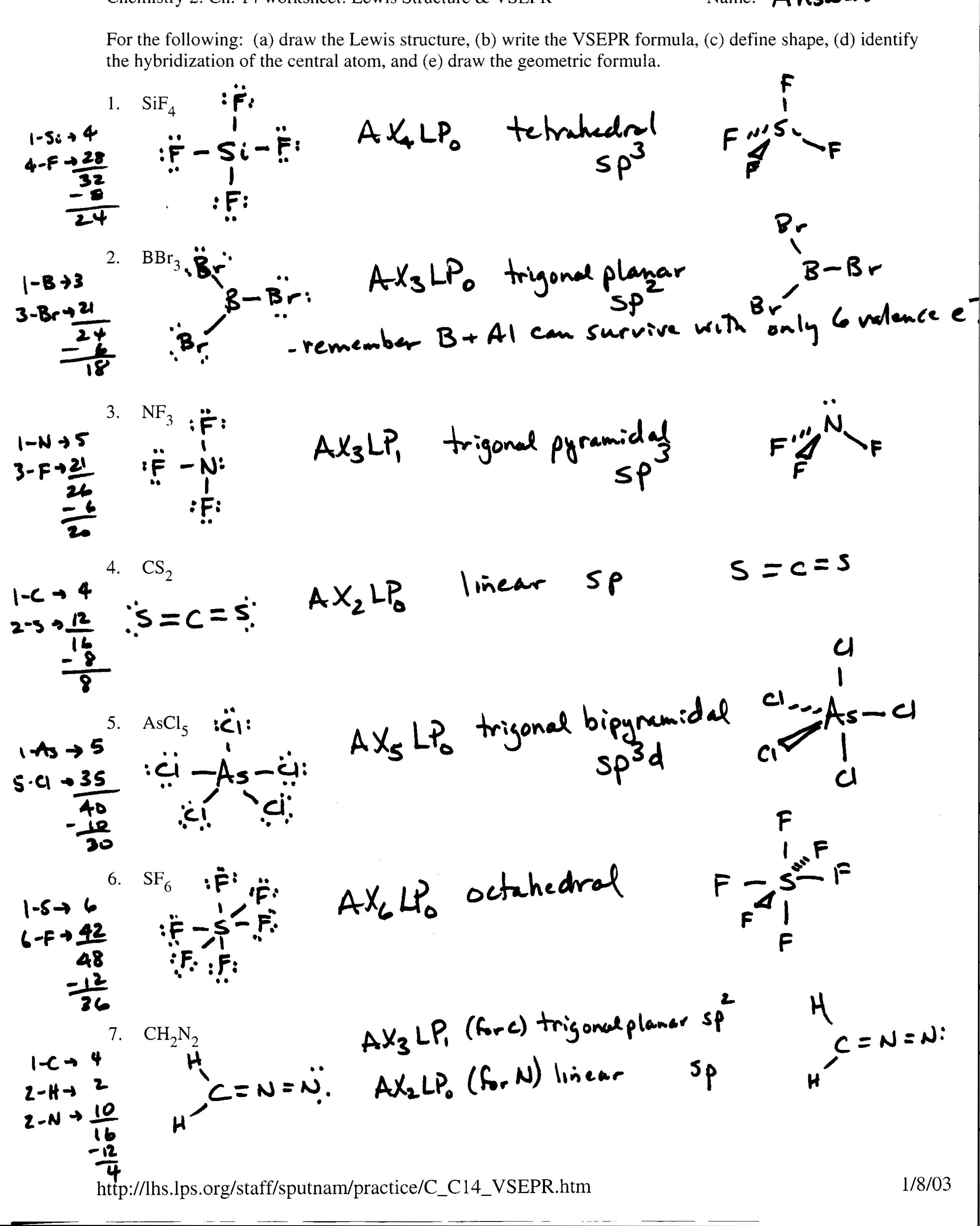
WEB Illustrate the formation of single double and triple covalent bonds using Lewis structures Student Lewis structures should show the sharing of a single pair of electrons two pairs of electrons and three pairs of electrons respectively for single
https://www.oneonta.edu/faculty/viningwj/Chem111
WEB bond properties bond order bond length bond energy bond polarity the shapes of molecules molecular polarity and how the shape and polarity of molecules influence chemical properties

https://quizlet.com/115687969
WEB Study with Quizlet and memorize flashcards containing terms like When sharing of electrons occurs the attachment between atoms is called in a covelant bond the dissociation energy is released in the process of When 2 or more atoms bond by means of electron sharing the result is and more

https://quizlet.com/390624167/chapter-8-covalent-bonding-flash-cards
WEB coordinate covalent bond a covalent bond in which one atom contributes both bonding electrons polyatomic ion a tightly bound group of atoms that behaves as a unit and has a positive or negative charge and behaves a unit bond dissociation energy the amount of energy required to break a covalent bond between atoms
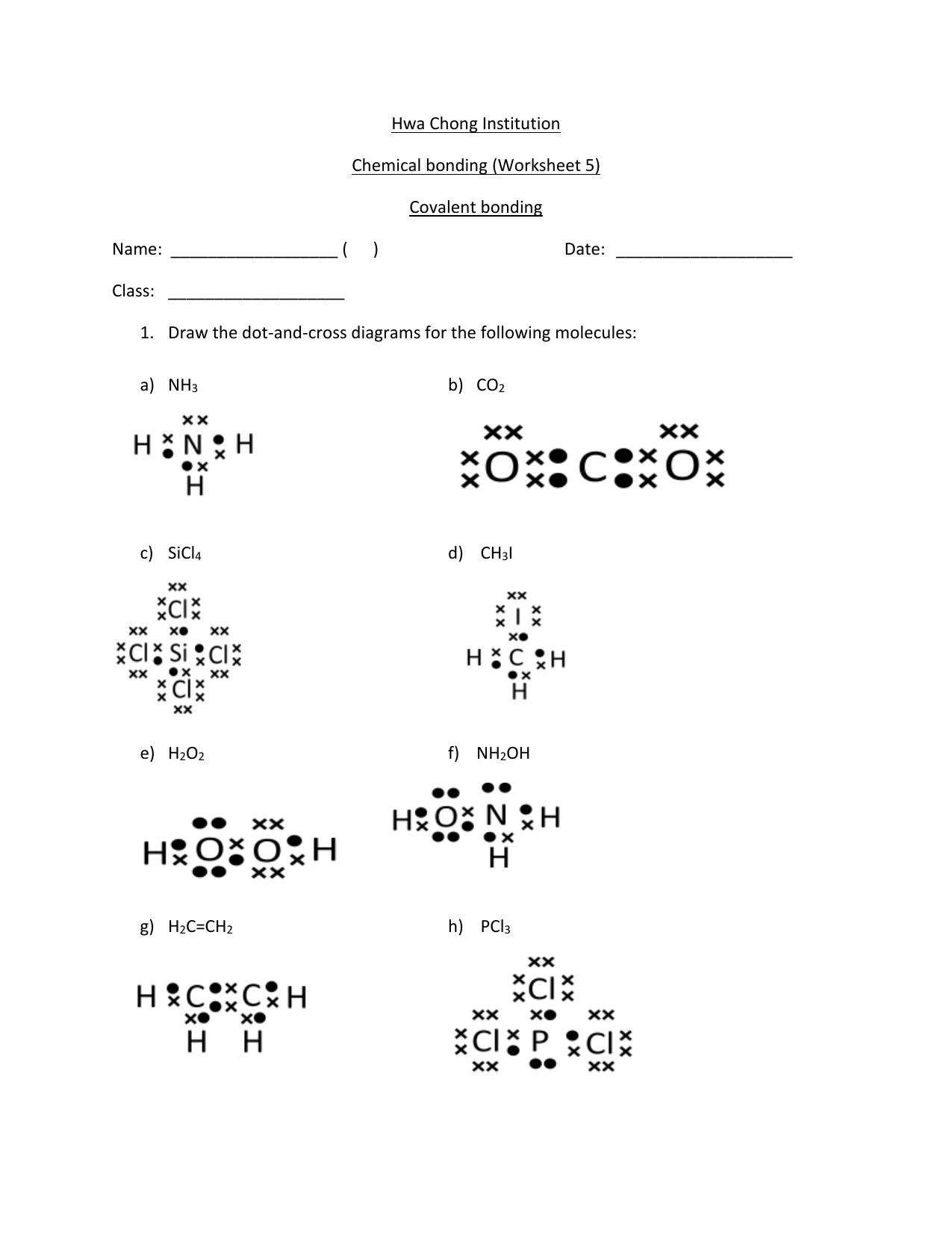
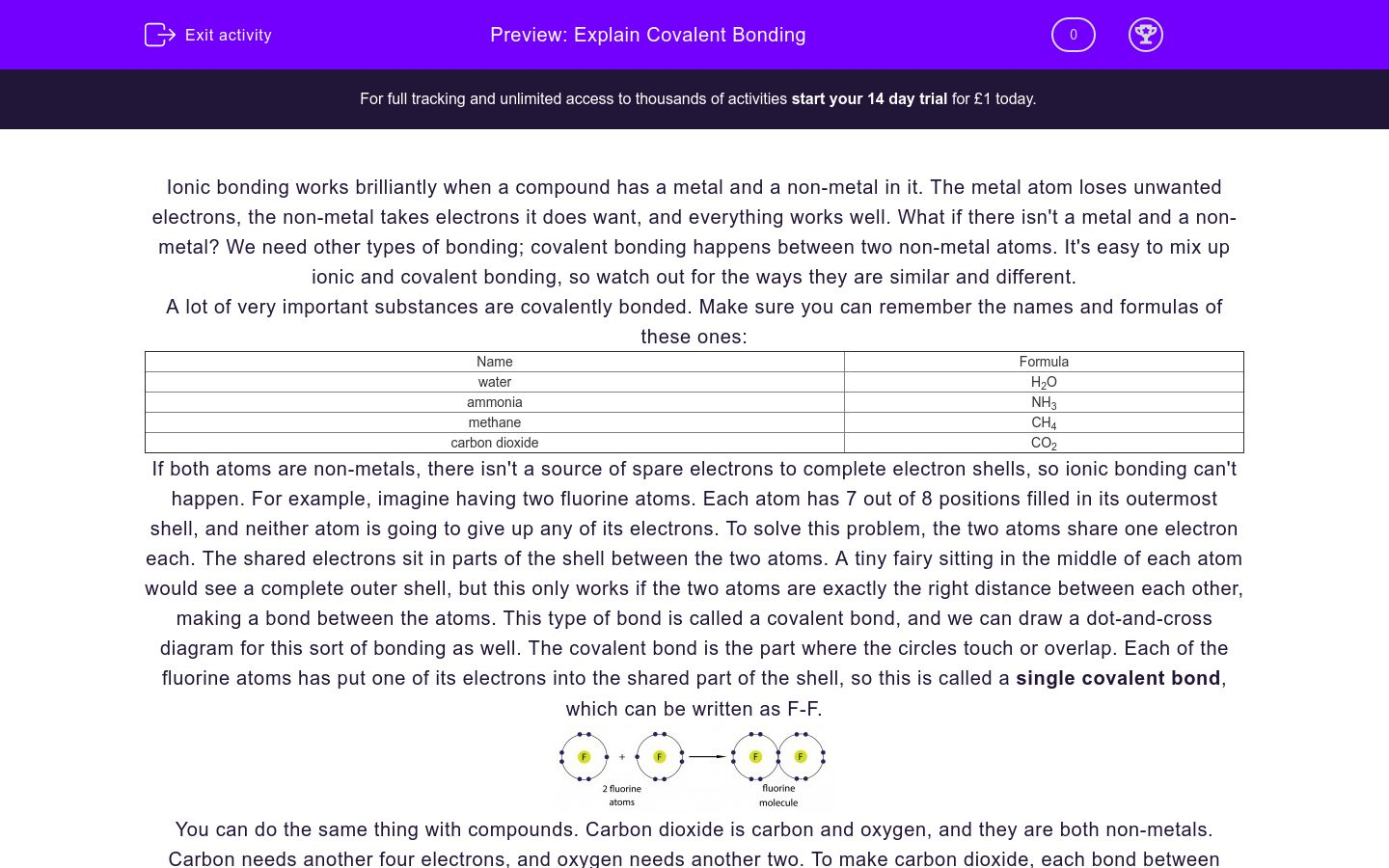
WEB COVALENT BONDING Name Covalent bonding occurs when two or more nonmetals share electrons attempting to attain a stable octet of electrons at least part of the time For example Note that hydrogen Is content with 2 not 8 electrons Show how covalent bonding occurs in each of the following pairs of atoms WEB Covalent bonding occurs when two or more NON METALS share electrons attempting to attain a stable octet 8 outer electrons in their outer shell for at least part of the time Draw a Lewis dot diagram for each element listed
WEB Mar 13 2023 0183 32 Learning Objectives Be able to define covalent bonds polar covalent bonds ionic bonds electronegativity dipoles formal charge molecular formula structural formula and electron dot formula Be able to recognize whether the type of bond between two atoms is covalent polar covalent or ionic