Determining Empirical Formulas True Formulas Worksheet Answers This document contains instructions to determine the empirical formula for 5 different compounds based on their elemental composition percentages or molecular weights For each compound the learner is asked to show their work in determining the empirical formula
Determining Empirical and Molecular Formulas Worksheet with Answers Free download as PDF File pdf or read online for free This document contains a worksheet with 15 questions about determining empirical and molecular formulas from combustion analysis data or percent composition by mass Students are asked to calculate empirical formulas determine if formulas represent empirical or molecular formulas calculate molecular formulas from molar masses write balanced
Determining Empirical Formulas True Formulas Worksheet Answers
 Determining Empirical Formulas True Formulas Worksheet Answers
Determining Empirical Formulas True Formulas Worksheet Answers
https://s3.studylib.net/store/data/008288647_1-6ac114b96b66925d490f5be78c7b879c.png
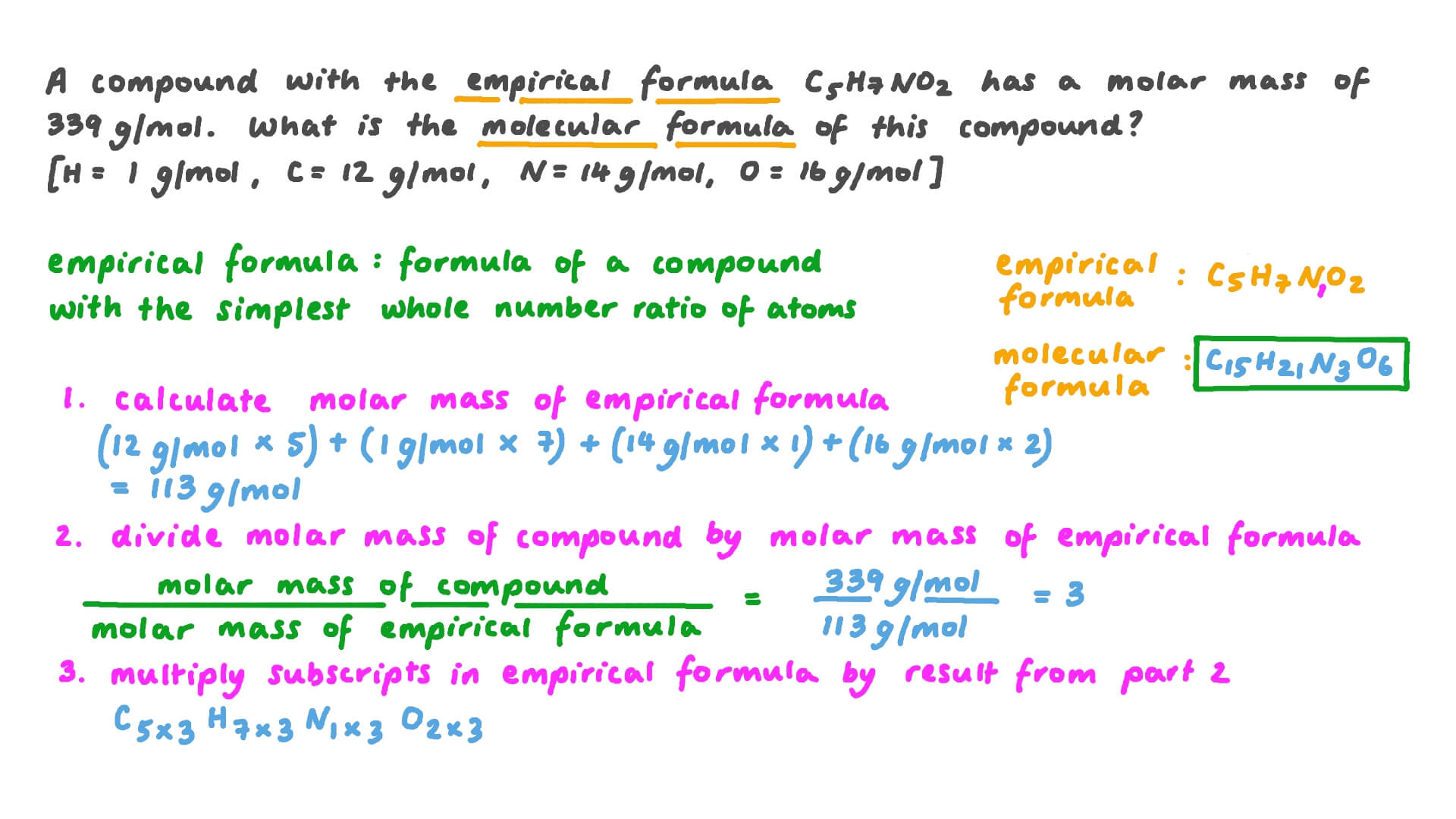
1 Distinguish between Empirical Formula and Molecular Formula 2 Determine a Molecular Formula given molecular mass and empirical formula for a compound 3 Students will be able to convert between empirical and molecular formulas thereby observing the usefulness of the different types of formulas Prerequisites Students should
Templates are pre-designed files or files that can be used for numerous functions. They can conserve time and effort by providing a ready-made format and design for developing different sort of material. Templates can be used for personal or professional projects, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
Determining Empirical Formulas True Formulas Worksheet Answers

Traditional Chemistry Ionic Formulas Worksheet

Empirical Formula And Molecular Formula Worksheet Igcse Free
Question Video Determining A Molecular Formula From An Empirical
Empirical Formulae 1 Chemsheets

Determining Empirical Formulas Worksheets
Empirical And Molecular Formulas Worksheet E streetlight

https://tsfx.edu.au › resources › W_-_Empirical
Write the empirical formula for the following compounds A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole What is the molecular formula of this compound

http://plaza.ufl.edu › ctoyota
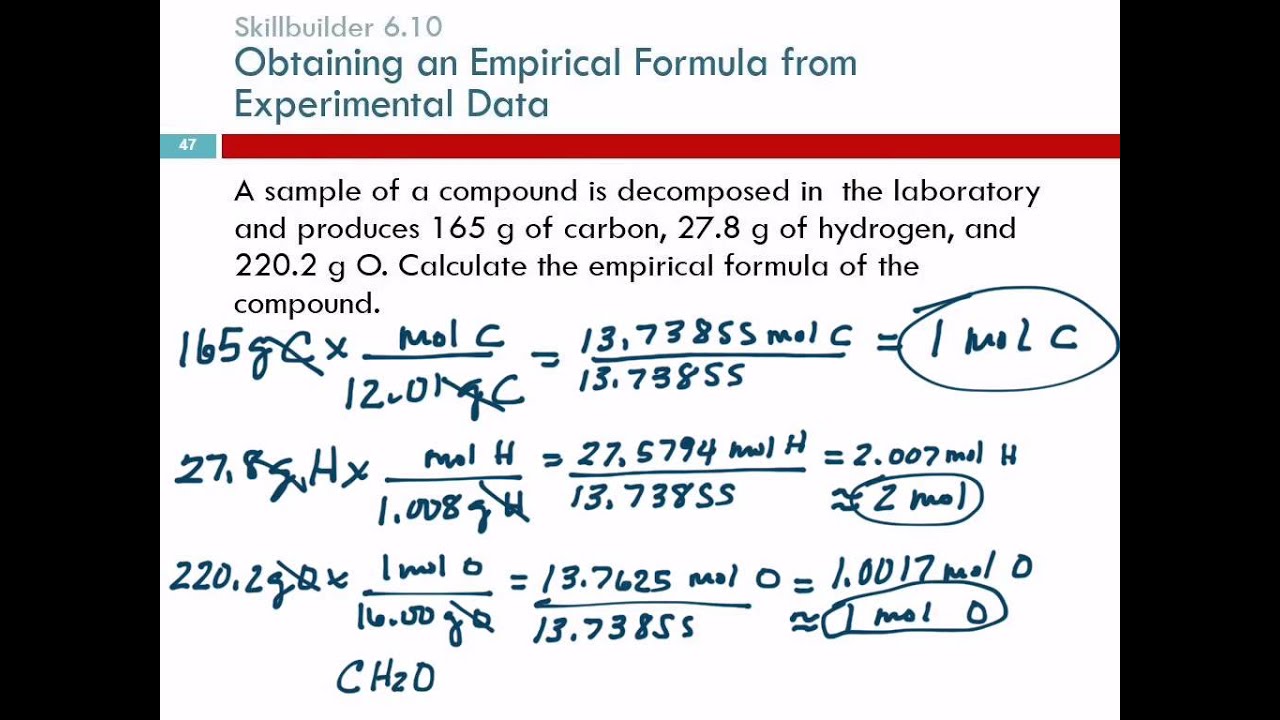
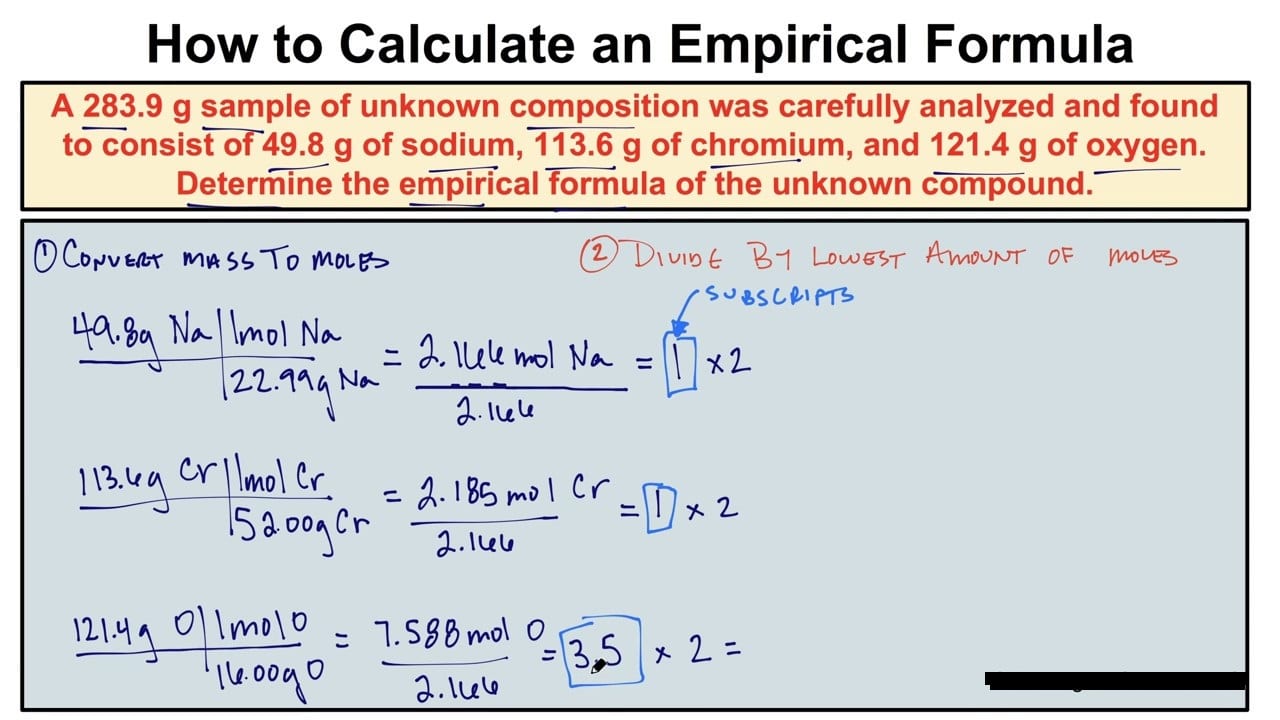
Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in the problems are grams Step 1 convert to moles Step 2 divide each by the lowest number of moles Step 3 only if necessary multiply all by the same factor in order to obtain whole numbers
.PNG?w=186)
https://hschemsolutions.com › files › Download
1 What is the empirical formula for C8H18 2 What is the empirical formula for H2O 3 What is the empirical formula for C4H10 4 What is the empirical formula for C2H4O2 5 Is CO2 an empirical formula a molecular formula or both Explain
http://colemanapchem.weebly.com › uploads ›
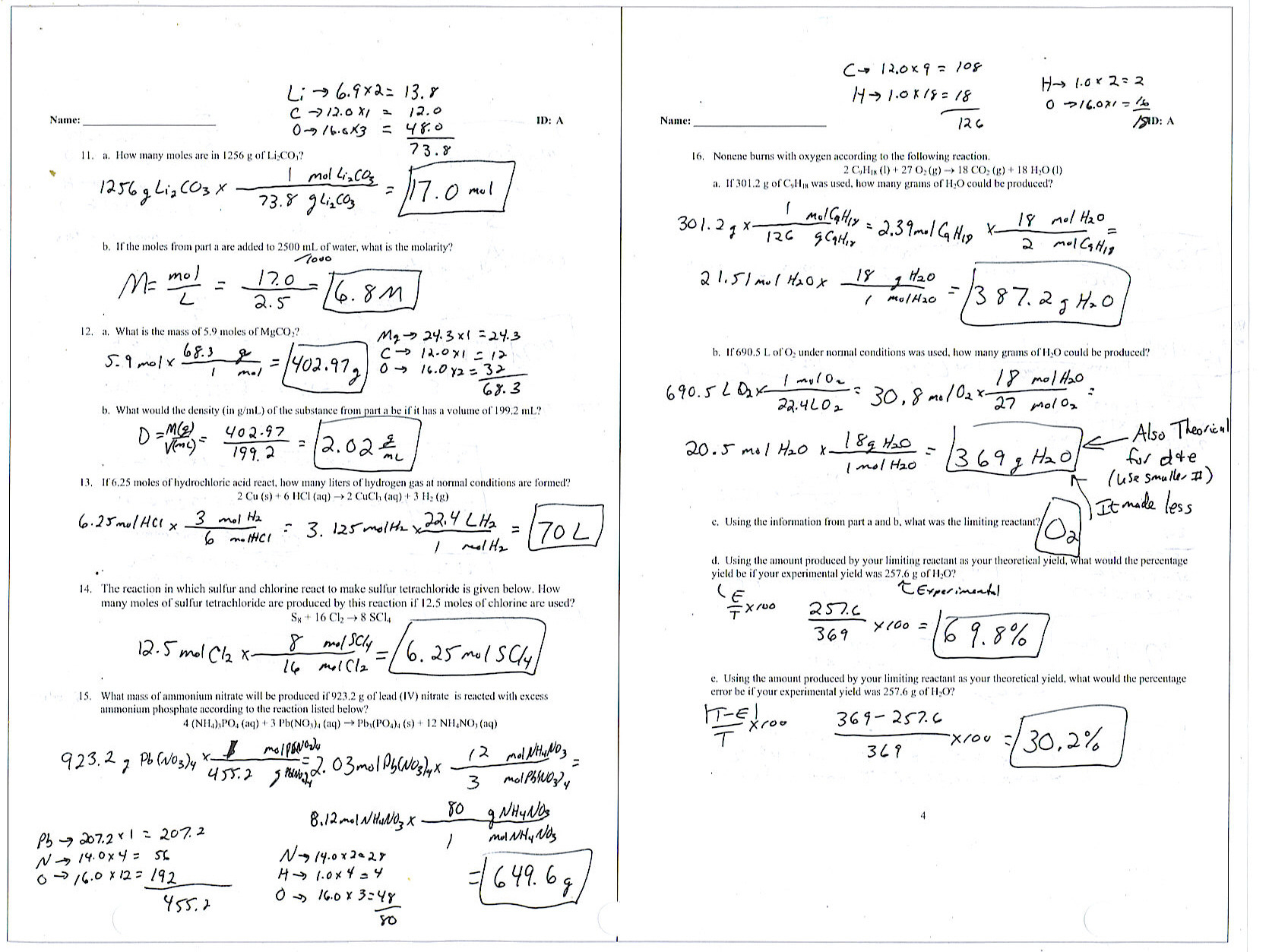
Determine the empirical formula of the compound 3 points 1 for masses to moles 1 for dividing by the smallest number of moles and 1 for writing the correct

https://www.studocu.com › en-us › document › st-louis
What is an empirical formula How is it different from a molecular formula The empirical formula is the lowest whole number ratio of atoms in a compound The molecular formula shows the true number of atoms in the molecule and is a whole number multiple of the empirical formula State the empirical formula for each of the following a C 6 H
Empirical and Molecular Formulas Worksheet Objectives be able to calculate empirical and molecular formulas Empirical Formula 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O These worksheets will guide them through determining the simplest whole number ratio of elements in a compound By working through various practice problems and exercises students will gain the skills and confidence needed to tackle empirical formula calculations easily
Empirical and Molecular Formula Worksheet 1 An unknown hydrocarbon contains 20 hydrogen and 80 carbon Find the empirical formula of this hydrocarbon If its molar mass is 30g what is its molecular formula EF MF 2 An unknown base contains 54 1 calcium 43 2 oxygen and 2 7 hydrogen What is this base s empirical formula