Difference Between Polarity And Electronegativity Dec 10 2017 0183 32 Chemistry 1 Answer Jose Dec 10 2017 Electronegativity occurs in elements and polarity in molecules Explanation ELEMENTS Electronegativity is the tendency of an atom to attract the electrons in a bond towards it MOLECULES Polarity means the separation of the charges
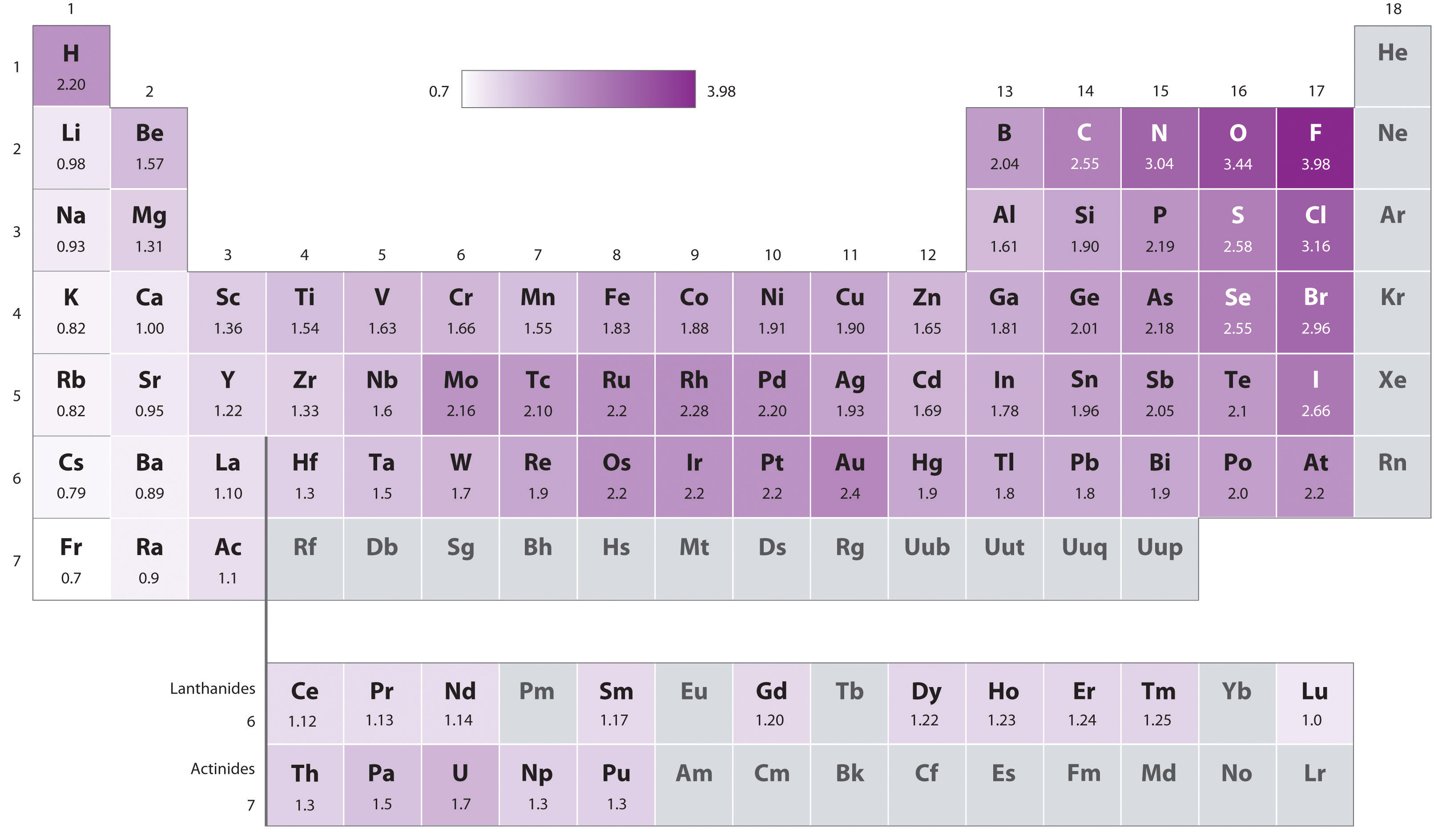
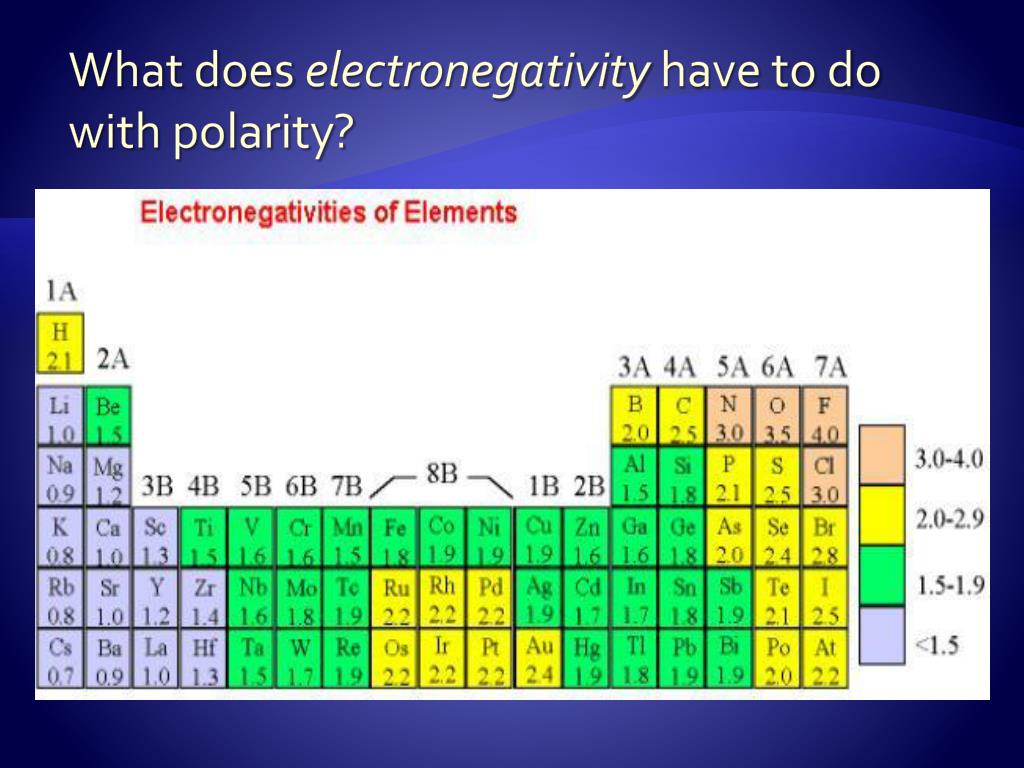
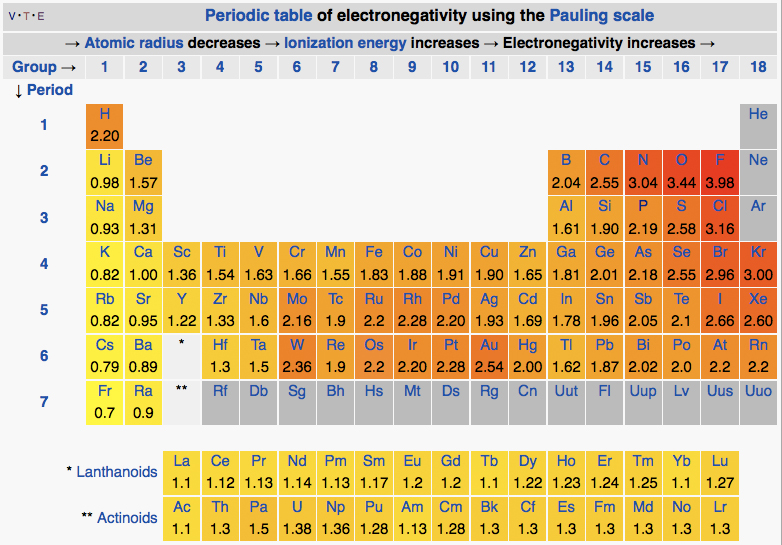
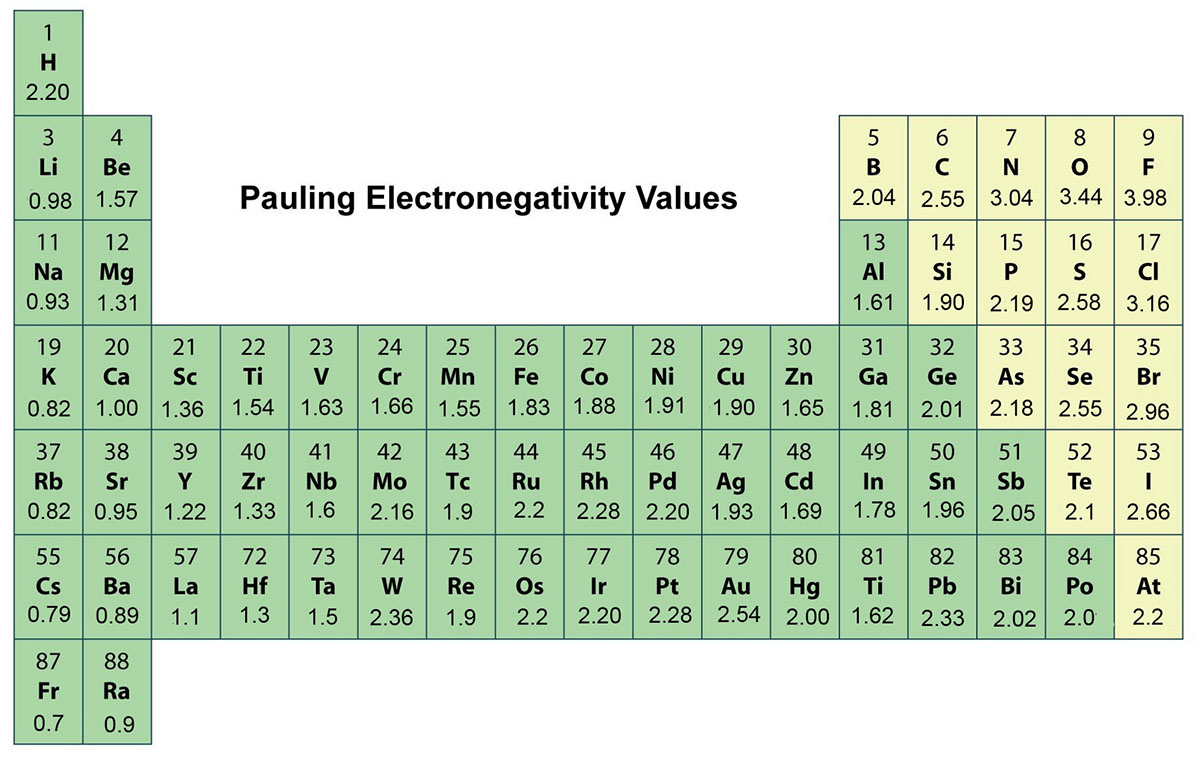
As a rough guide bonds between atoms whose electronegativities differ by less than 0 5 are nonpolar covalent bonds between atoms whose electronegativities differ by 0 5 to 2 are polar covalent and bonds between atoms whose electronegativities differ by more than 2 are largely ionic So the difference in electronegativity is somewhere between 1 5 and 2 1 between a polar covalent bond and an ionic bond So most textbooks we ll see approximately somewhere around 1 7 So if you re higher than 1 7 it s
Difference Between Polarity And Electronegativity
 Difference Between Polarity And Electronegativity
Difference Between Polarity And Electronegativity
http://images.flatworldknowledge.com/averillfwk/averillfwk-fig08_011.jpg
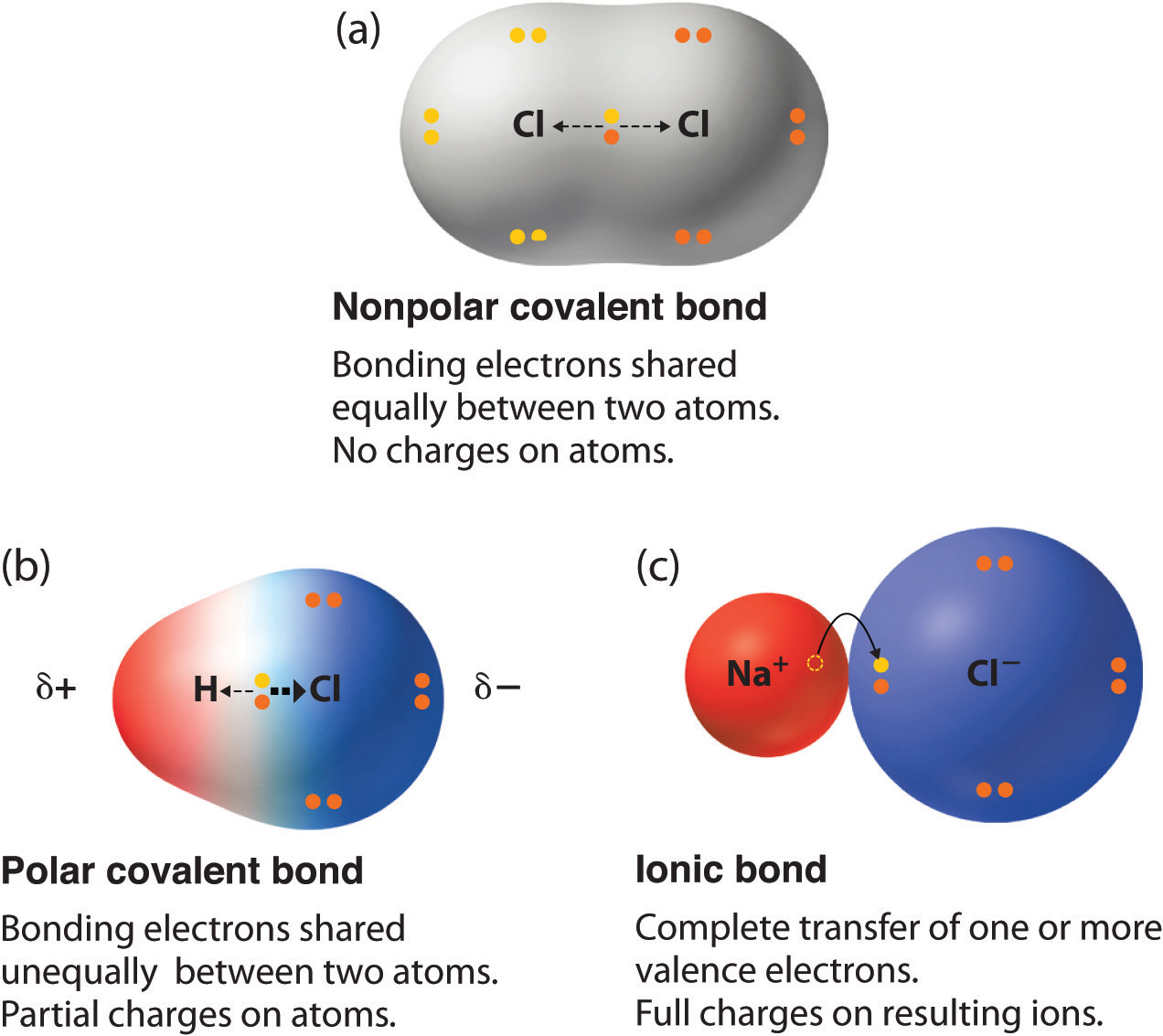
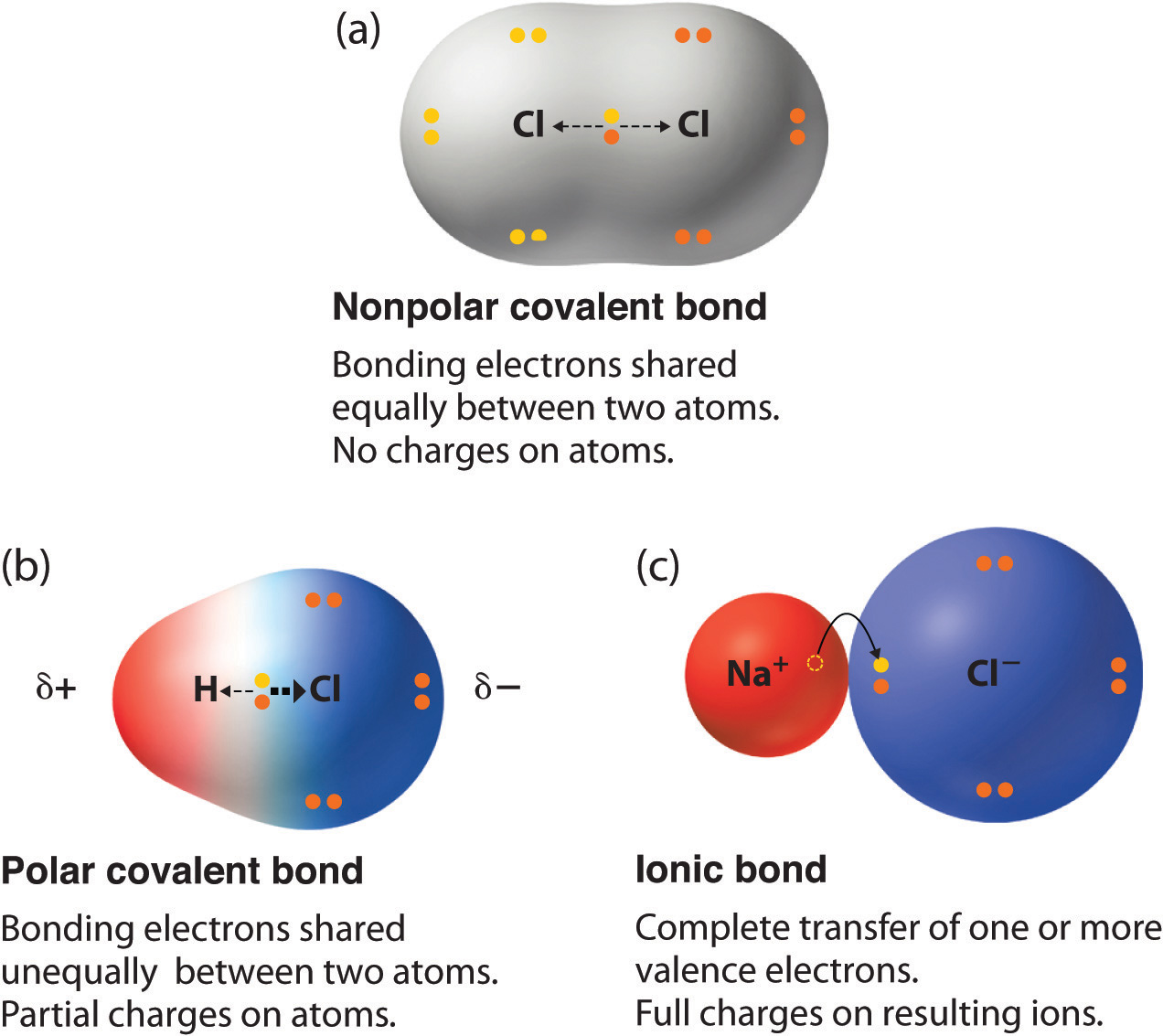
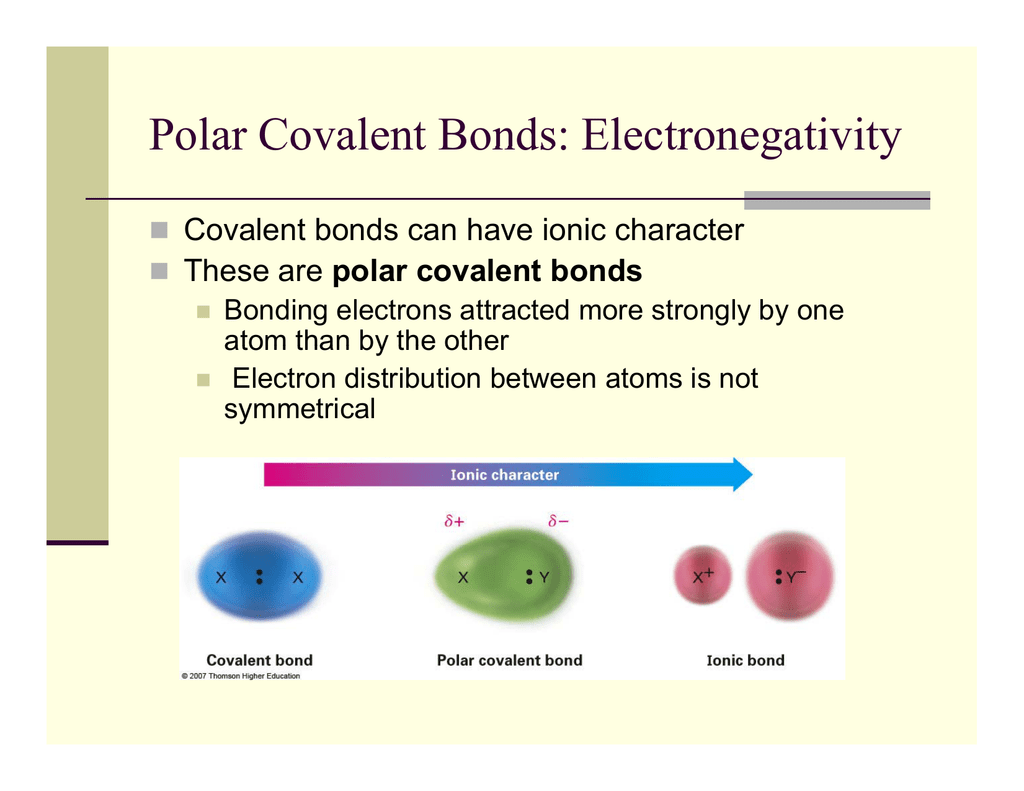
The polarity of a covalent bond can be judged by determining the difference in the electronegativities of the two atoms making the bond The greater the difference in electronegativities the greater the imbalance of electron sharing in the bond Although there are no hard and fast rules the general rule is
Templates are pre-designed files or files that can be utilized for various functions. They can conserve time and effort by providing a ready-made format and layout for producing various sort of content. Templates can be used for personal or expert tasks, such as resumes, invites, leaflets, newsletters, reports, presentations, and more.
Difference Between Polarity And Electronegativity

Question Video Determining Which System Has The Greatest Difference Of
8 4 Bond Polarity And Electronegativity Chemistry LibreTexts
PPT Bond Polarity And Molecules PowerPoint Presentation Free
Difference Between Electronegativity And Polarity Compare The
Electronegativity Explained

What Is Electronegativity Chart List Of Electronegativity PDF
https://chem.libretexts.org/Courses/Oregon
The absolute value of the difference in electronegativity EN of two bonded atoms provides a rough measure of the polarity to be expected in the bond and thus the bond type When the difference is very small or zero the bond is covalent and nonpolar When it is large the bond is polar covalent or ionic

https://chem.libretexts.org/Courses/Anoka-Ramsey
Apr 16 2023 0183 32 A bond in which the electronegativity difference between the atoms is between 0 4 to 1 9 is called a polar covalent bond A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons so the electrons are shared unequally

https://thisvsthat.io/electronegativity-vs-polarity
Molecular shape bond type and electronegativity difference between atoms affect polarity Periodic Trend Electronegativity generally increases across a period from left to right on the periodic table Polarity can vary across different compounds and is not strictly tied to periodic trends Bond Type
https://pressbooks.online.ucf.edu//chapter/electronegativity-and-polarity
The absolute value of the difference in electronegativity EN of two bonded atoms provides a rough measure of the polarity to be expected in the bond and thus the bond type When the difference is very small or zero the bond is covalent and nonpolar When it is large the bond is polar covalent or ionic

https://chemguide.co.uk/atoms/bonding/electroneg.html
Definition Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons The Pauling scale is the most commonly used Fluorine the most electronegative element is assigned a value of 4 0 and values range down to caesium and francium which are the least electronegative at 0 7
Jul 26 2021 0183 32 chemistry The tendency or a measure of the ability of an atom or molecule to attract electrons when forming bonds Jul 26 2021 Polarity An indicated polar extreme An electric terminal with positive polarity Jul 26 2021 Electronegativity chemistry the tendency of an atom or radical to attract electrons in the formation of an ionic bond Dec 2 2023 0183 32 Key Differences Electronegativity is a chemical property that describes how strongly an atom can attract or hold onto electrons In contrast polarity is a broader concept that refers to the distribution of electric charges in a molecule determined by the arrangement of atoms and the electronegativity of each atom Sara Rehman Dec 02
In general Electronegativity differences less than 0 5 are considered to be nonpolar covalent bonds Electronegativity differences between 0 5 and 1 9 are considered increasingly polar covalent bonds Electronegativity differences of 2 or greater than 2 are considered to be ionic bonds The higher the polarity of a bond the stronger the bond