Endothermic And Exothermic Reactions Lab Worksheet Note Decomposition reactions use up energy in the form of light heat or electricity Therefore they are called endothermic reactions since they use up or absorb energy Displacement
Decomposition reactions are usually endothermic because they involve breaking of bonds Breaking bonds requires an input of energy while forming bonds releases energy Since a Endothermic reactions are those reactions in which heat is absorbed Reactants heat products displaystyle Reactants heat rightarrow products R e a c t a n t
Endothermic And Exothermic Reactions Lab Worksheet
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png) Endothermic And Exothermic Reactions Lab Worksheet
Endothermic And Exothermic Reactions Lab Worksheet
https://www.thoughtco.com/thmb/LC9VMDICnwzLlDS9bUzWoGroMRM=/3000x2000/filters:fill(auto,1)/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png
The process of respiration is a an oxidation reaction which is endothermic b a reduction reaction which is exothermic
Templates are pre-designed files or files that can be used for various functions. They can save time and effort by supplying a ready-made format and layout for producing different kinds of material. Templates can be used for personal or expert jobs, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
Endothermic And Exothermic Reactions Lab Worksheet

Endothermic And Exothermic Reactions lab Based Worksheet KS3
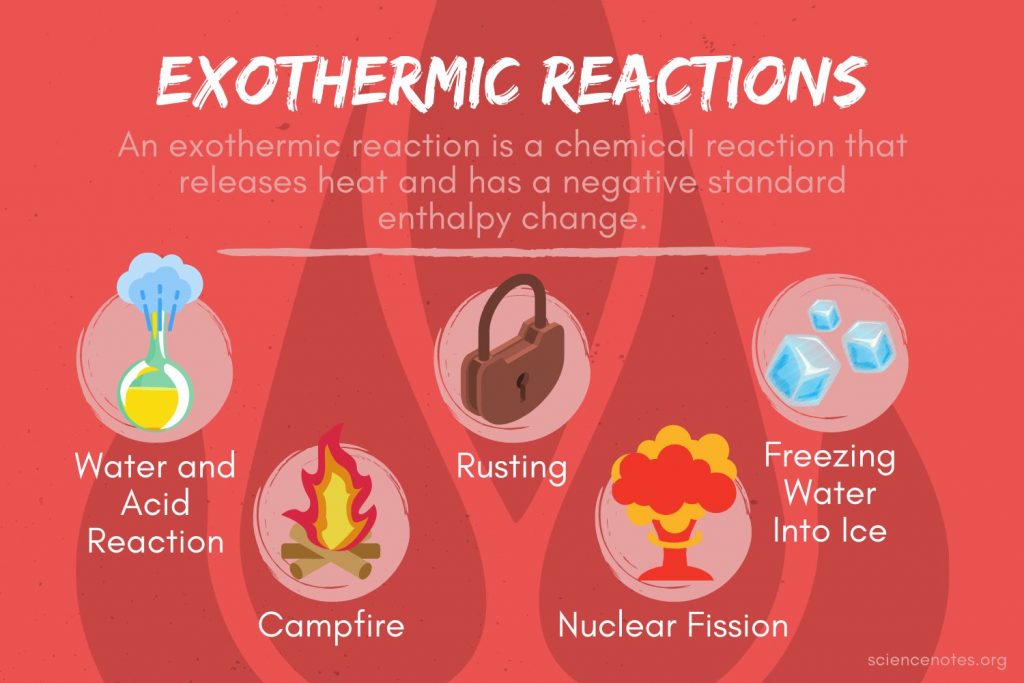
What Does One Mean By Exothermic And Endothermic Reactions Give
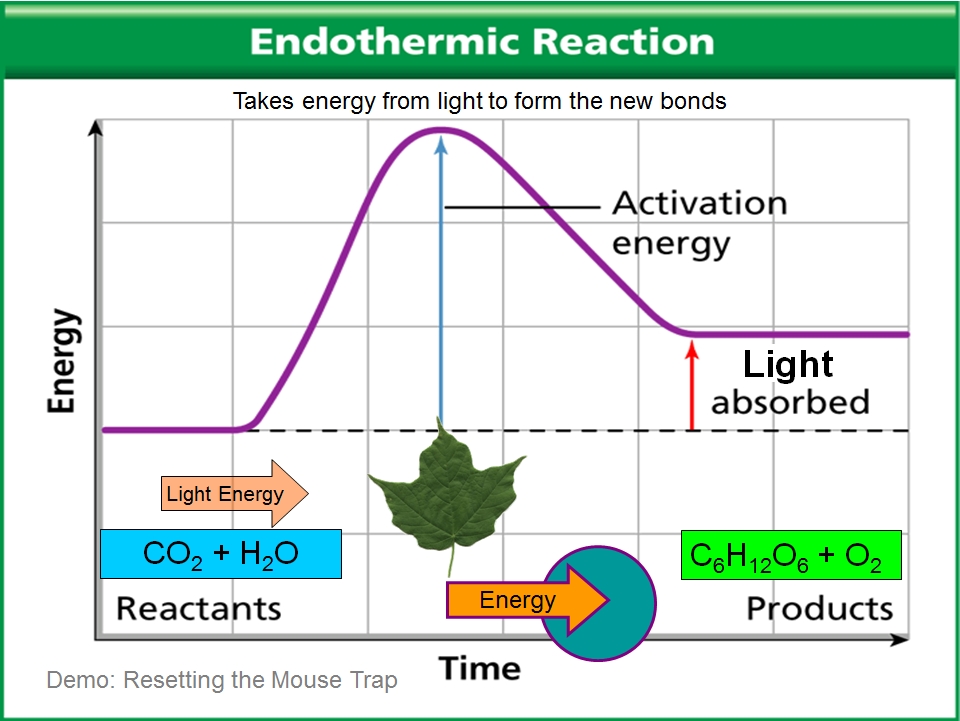
Endo Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE

10 Endothermic Reactions Vs Exothermic Reactions Worksheet
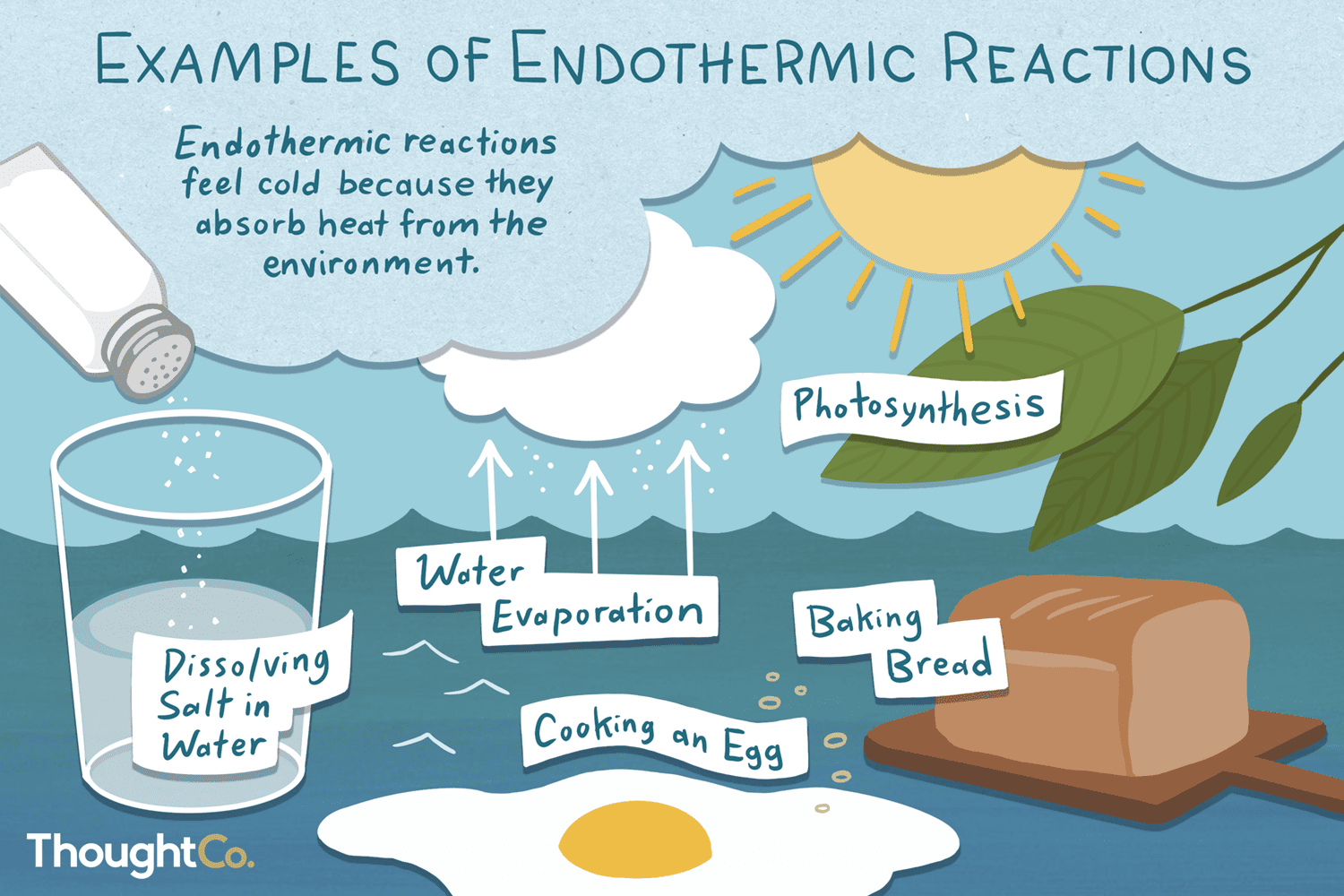
What Does One Mean By Exothermic And Endothermic Reactions Give

Endothermic And Exothermic Reactions LAB 01
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png?w=186)
https://www.toppr.com › guides › biology › difference-between › endothe…
The endothermic process is a term which describes a chemical reaction where the system absorbs the heat energy from its surrounding Some such examples are evaporating liquids

https://www.toppr.com › ask › question › why-is-photosynthesis-consider…
a What do you understand by exothermic and endothermic reactions b Give one example of an exothermic reaction and one of an endothermic reaction c Which of the following are

https://www.toppr.com › ask › question › explain-endothermic-reaction-w…
a Give an example of an oxidation reaction b Is oxidation an exothermic or an endothermic reaction c Explain by giving an example how oxidation and reduction proceed side by side

https://www.toppr.com › ask › question
Water is a higher energy state as the liquid can rotate and vibrate while solid ice can only vibrate This means for ice to turn into a higher energy state water it has to absorb energy hence it is
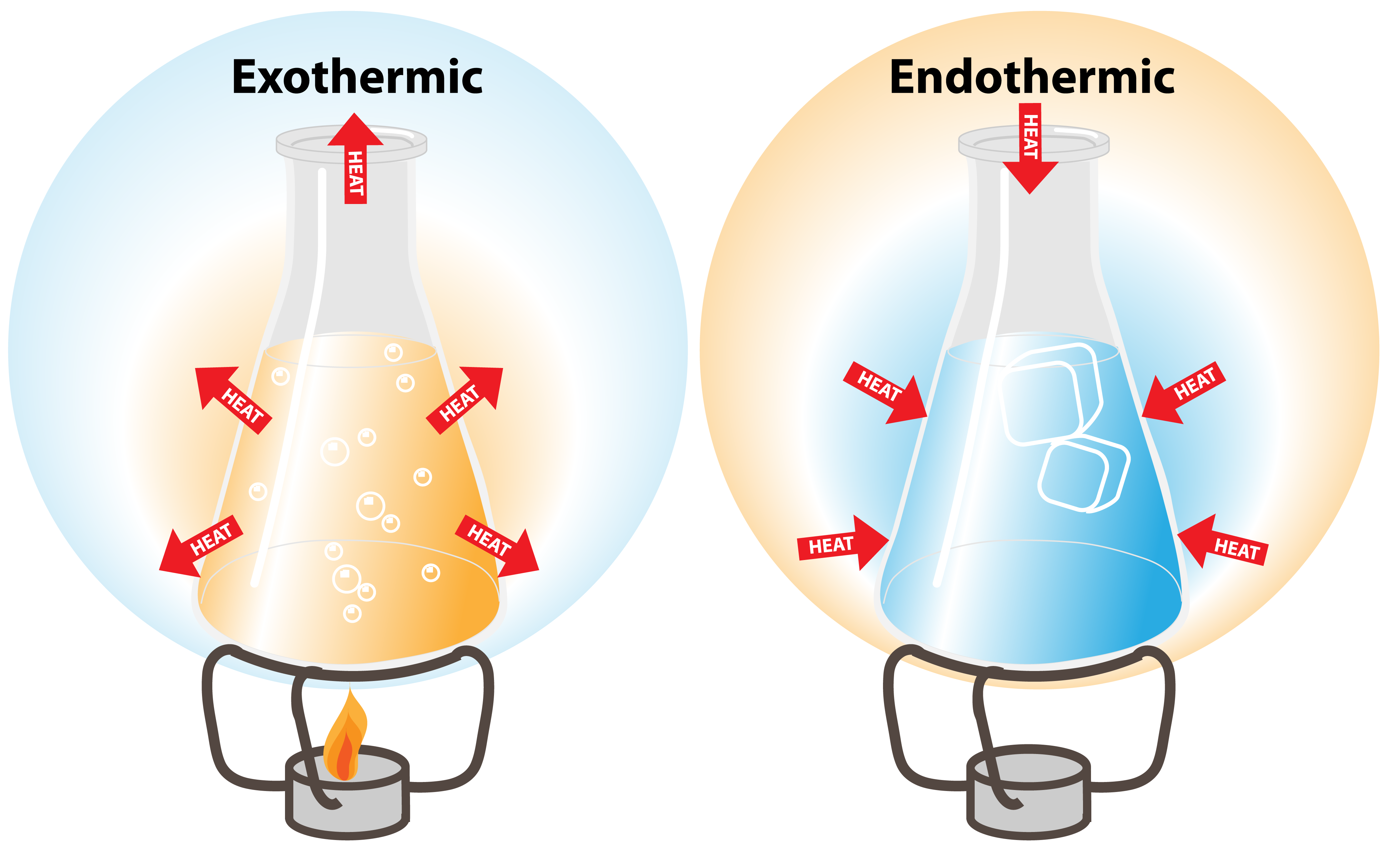
https://www.toppr.com › › is-the-freezing-of-water-an-exothermic-or-a…
An endothermic process would imply that heat must be supplied to the system That is clearly not the case here since providing heat would actually increase the average kinetic energy of the
[desc-11] [desc-12]
[desc-13]