Flame Test Lab Conclusion Answers Nov 7 2019 0183 32 When a substance such as an unknown metal or metalloid is put into a flame the heat of the flame excites the electrons When electrons are excited they go from ground state
In this experiment you will perform the flame tests used to identify several metallic elements SAFETY FIRST In this lab the solutions you will be using contain harmful materials Avoid Study with Quizlet and memorize flashcards containing terms like What is a flame test What does it show How is it done Under what circumstances do atoms give off light Why do atoms of different elements give off different colors of
Flame Test Lab Conclusion Answers
Flame Test Lab Conclusion Answers
http://4.bp.blogspot.com/-R_aGnlGfB28/VZ5zznrkjyI/AAAAAAAAAIo/fYik88QLugI/s1600/photo%2B%25285%2529.JPG
May 21 2024 0183 32 During a flame test elements are heated and their electrons of the atoms gain energy from the flame When they lose this energy they emit colors of visible light Since each
Templates are pre-designed documents or files that can be used for various functions. They can save time and effort by offering a ready-made format and layout for creating different type of content. Templates can be used for personal or professional jobs, such as resumes, invitations, leaflets, newsletters, reports, discussions, and more.
Flame Test Lab Conclusion Answers
Flame Test Lab Results

Flame Test Lab YouTube

Flame Test Sequence Photograph By Pixels

Lab Report Conclusion Template Atlanticcityaquarium

Drawing Conclusions Examples With Answers Writing Conclusion
Flame Test Lab Answer Key
https://gradesfixer.com › › flame-test-lab-conclusion
Mar 20 2024 0183 32 The flame test lab allowed us to draw several conclusions about the behavior of metal ions in the presence of a flame Firstly it demonstrated the unique emission spectra of
:max_bytes(150000):strip_icc()/GettyImages-680790185-58979cfc3df78caebc1b69db.jpg?w=186)
https://quizlet.com › flame-tests-lab
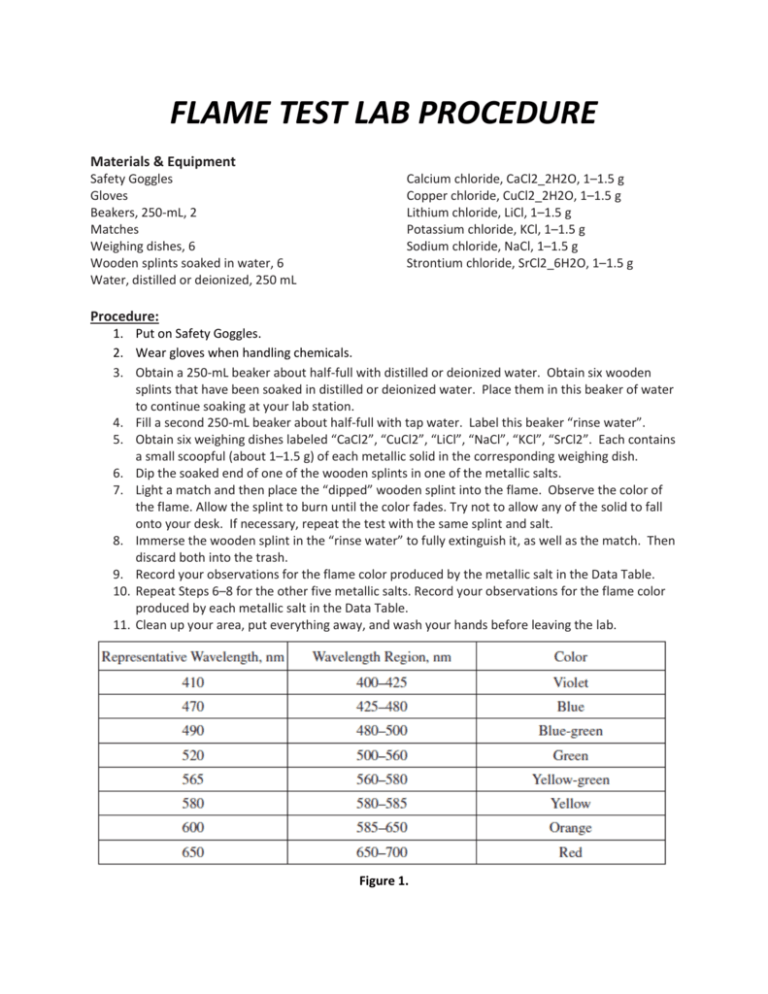
Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the metal ion present in one unknown metal salt solution Independent variable metal used dependent

https://www.coursehero.com › file › Flame
Mar 24 2020 0183 32 Flame Test Lab Conclusion Questions 1 Is there a correlation between the color of the salt and the color of the flame There wasn t a correlation between the color of the salt and the color of the flame
https://www.studocu.com › › chemistry
QUESTIONS AND ANSWERS Several compounds contain the Cl anion a Do these compounds have the same flame color b What about compounds containing the SO 4 2 anion c The CO 3 2 anion d So is the anion

https://msrandallscience.weebly.com › uploads ›
Lab Conclusion Flame Test 1 Write a paragraph summarizing what you have learned about the scientific concept of the lab from doing the lab Back up your statement with details from your
In a flame test experiment Q tips coated with different elemental crystals were placed in a Bunsen burner flame The elements absorbed energy and excited electrons to higher energy levels Based on the experimental results it is safe to conclude that various elements display different colors when exposed to a flame and the presence of these colors is evidence of atomic
To get started describe the goal of the flame test lab in identifying the various metals based on the color of the flame produced when the metal ions are excited by heat The purpose of the