How To Convert Masses Into Moles May 9 2010 0183 32 Converting Mass to Moles Melanie Lee 49 subscribers Subscribe 245 Share Save 72K views 13 years ago an explanation of how to use dimensional analysis
Molar mass g mol Moles How to Convert Mass to Mole In chemistry the molar mass of a chemical compound is defined as the mass of 1 mole or 6 02214 215 10 23 particles of the substance expressed in grams Dec 5 2023 0183 32 Method 1 Calculating the Molar Mass of an Element Download Article 1 Understand molar mass Molar mass is the mass in grams of one mole of a substance 3 Using the atomic mass of an element and multiplying it by the conversion factor grams per mole g mol you can calculate the molar mass of that element 2
How To Convert Masses Into Moles
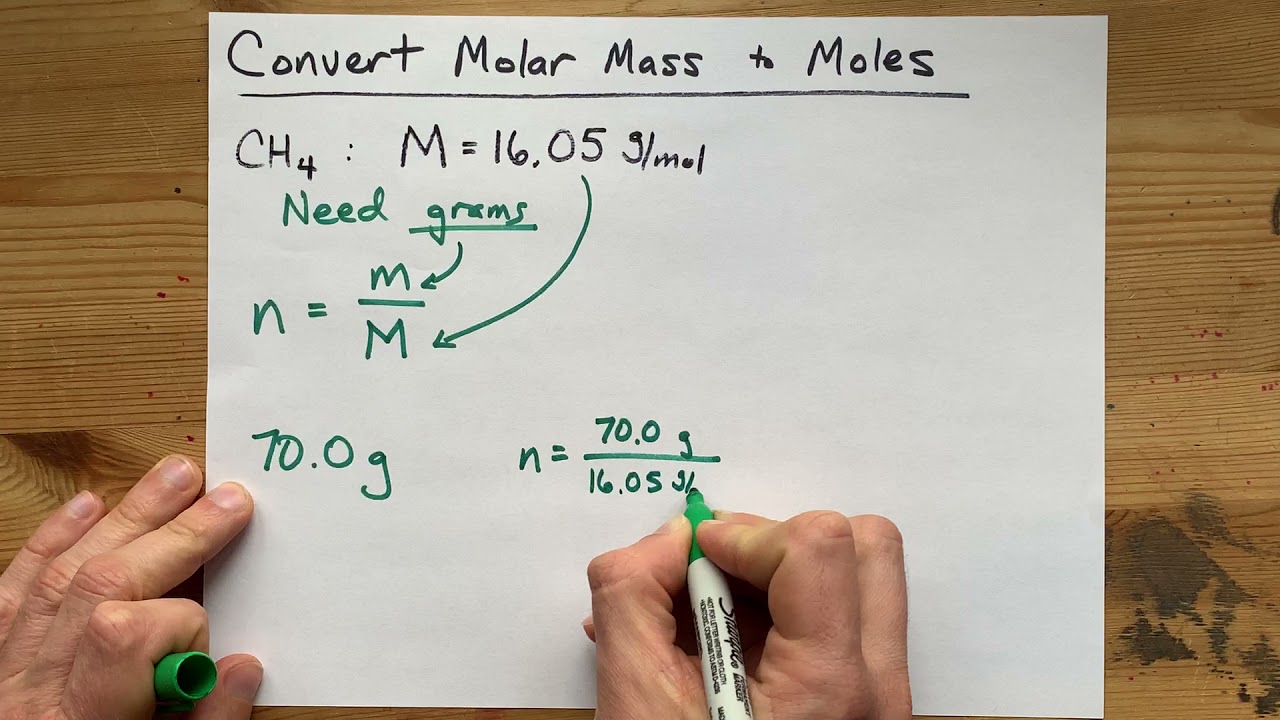 How To Convert Masses Into Moles
How To Convert Masses Into Moles
https://i.ytimg.com/vi/JbmCTKv0n6k/maxresdefault.jpg
Jan 18 2024 0183 32 m 6 l 215 998 kg m 179 0 006 m 179 215 998 kg m 179 5 988 kg It s easier to work with grams so convert the mass 5 988 kg 5988 g As you already know how the grams to moles conversion work find the number of moles n 5988 g 18 015 g mol 332 4 mol You can always use our grams to moles calculator to check the result
Pre-crafted templates use a time-saving service for producing a varied variety of files and files. These pre-designed formats and designs can be used for different individual and expert tasks, consisting of resumes, invites, flyers, newsletters, reports, presentations, and more, streamlining the material creation process.
How To Convert Masses Into Moles
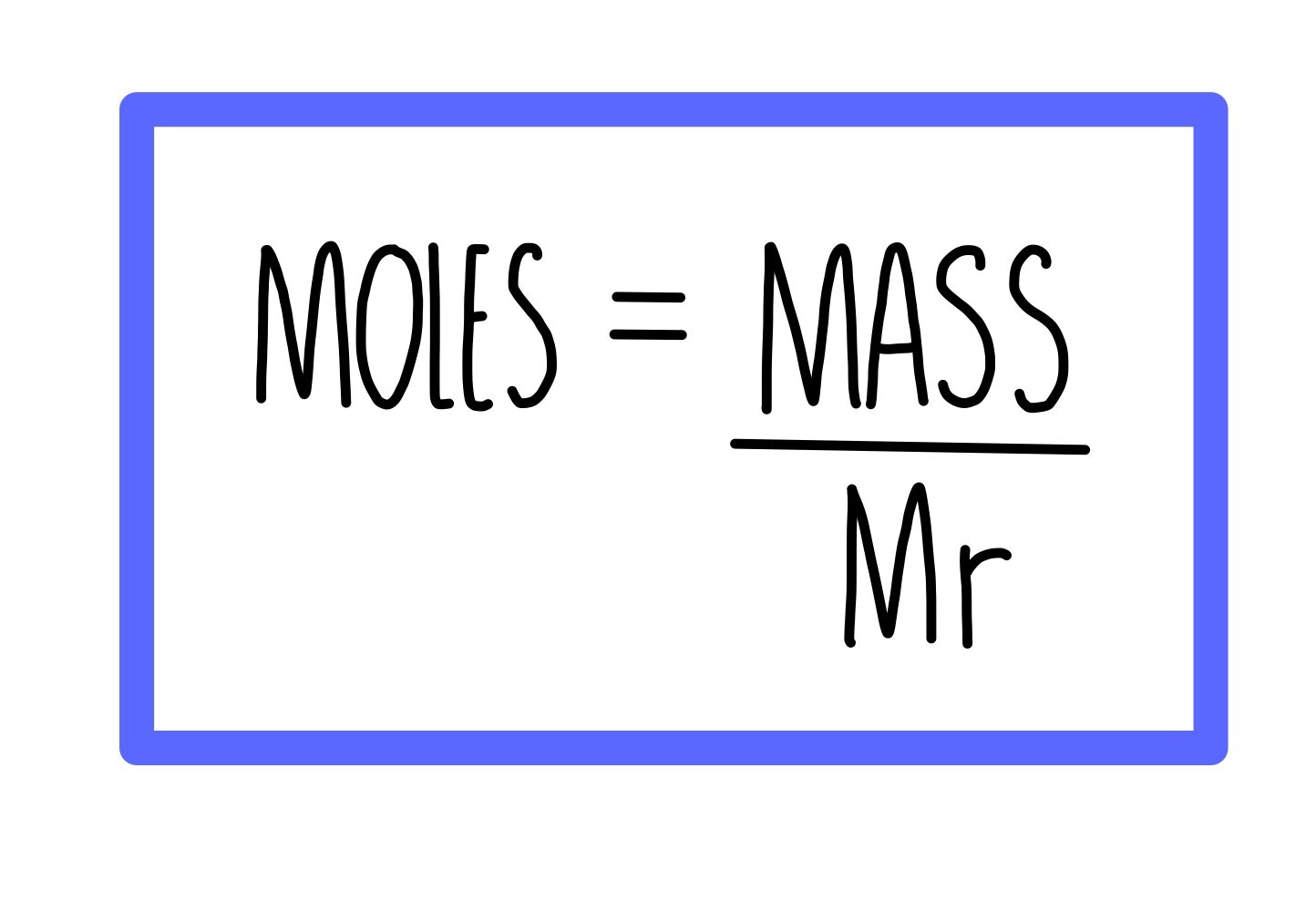
Concentration And Moles AQA The Science Sauce

Moles To Particles Calculator Tewsgw

Mass To Mole Conversions YouTube
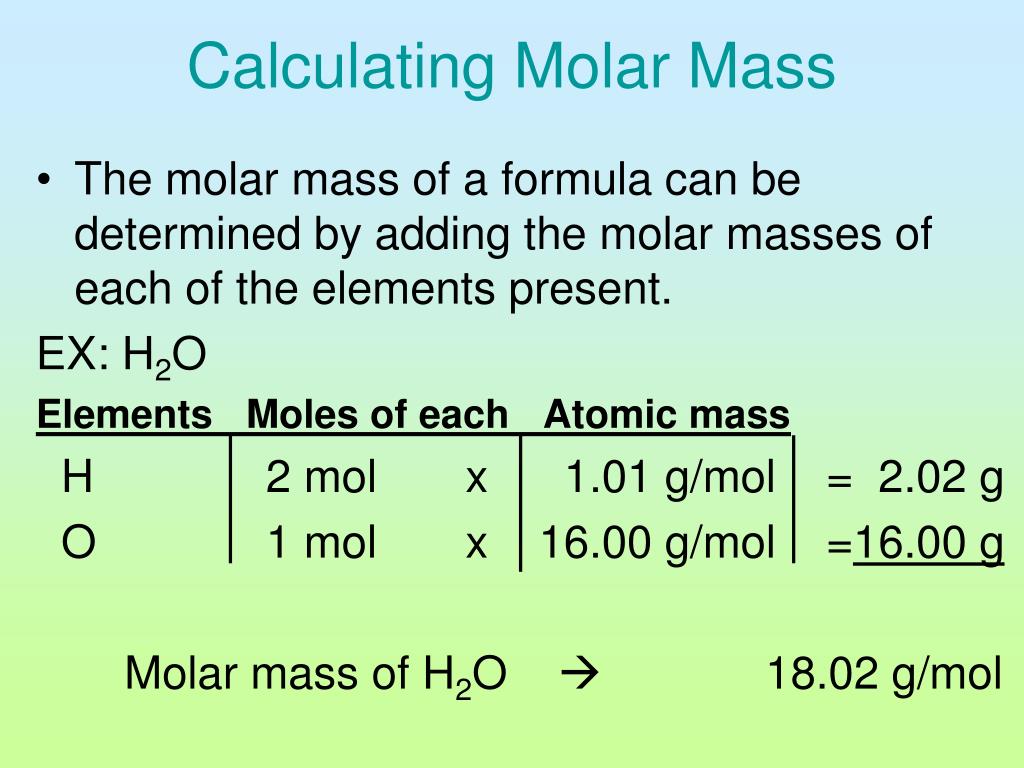
How To Calculate Density From Molecular Weight Haiper

Mole Conversion Worksheet And Activity ITeachly
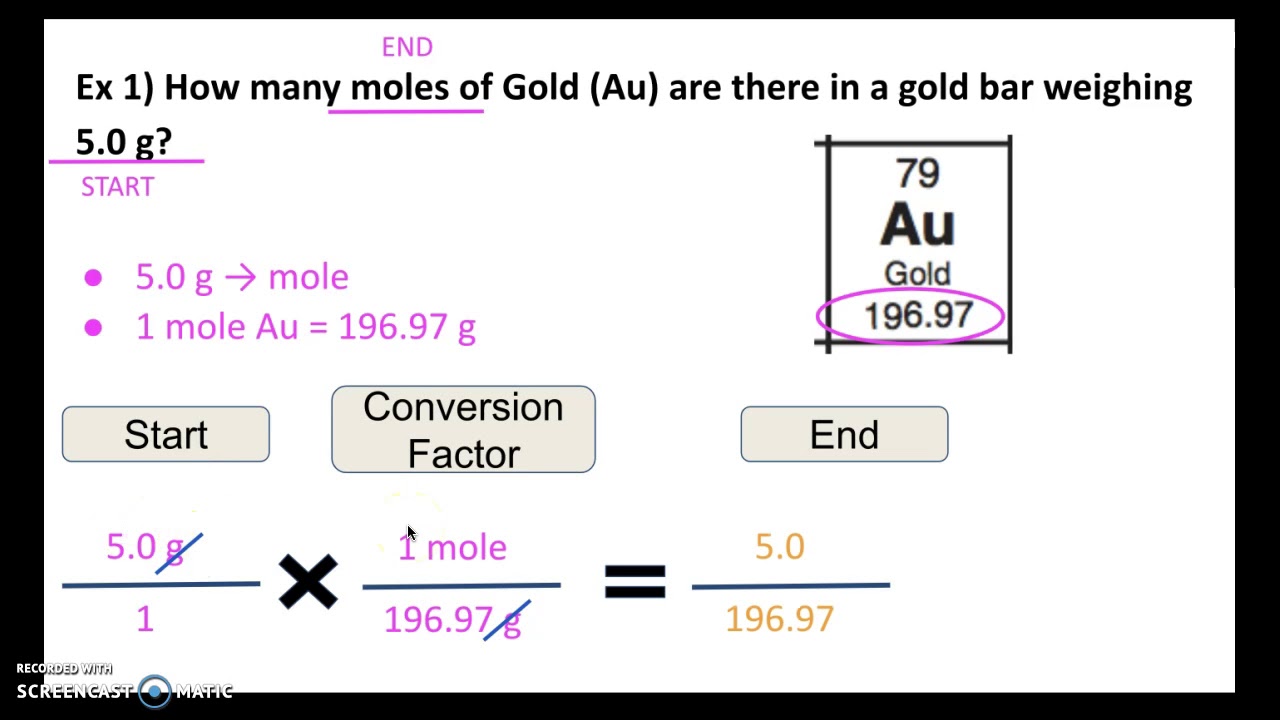
Converting Moles Mass Part 1 Of 2 YouTube

https://chem.libretexts.org/Bookshelves
Aug 10 2022 0183 32 If we start with a known mass of one substance in a chemical reaction instead of a known number of moles we can calculate the corresponding masses of other substances in the reaction The first step in this case is to convert the known mass into moles using the substance s molar mass as the conversion factor

https://chem.libretexts.org/Bookshelves
The molar mass of ce CaCl 2 is 110 98 text g mol The conversion factor that can be used is then based on the equality that 1 text mol 110 98 text g ce CaCl 2 Dimensional analysis will allow you to calculate the mass of ce CaCl 2 that you should measure

https://chem.libretexts.org/Courses/Portland
Jul 28 2022 0183 32 If we start with a known mass of one substance in a chemical reaction instead of a known number of moles we can calculate the corresponding masses of other substances in the reaction The first step in this case is to convert the known mass into moles using the substance s molar mass as the conversion factor
https://www.wikihow.com/Convert-Grams-to-Moles
Aug 21 2023 0183 32 Using a calculator divide the number of grams by the molar mass The result is the number of moles in your element or compound For example imagine you have 2 g of NH 4 2 S and you want to convert it to moles The molecular mass of NH 4 2 S is 68 17g mol Divide 2 by 68 17 and you have 0 0293 moles of NH 4 2 S

https://www.thoughtco.com/convert-grams-to-moles
Jun 4 2022 0183 32 Using the factor 1 mol 44 01 g moles CO 2 454 g x 1 mol 44 01 g 10 3 moles Answer There are 10 3 moles of CO 2 in 454 grams of CO 2 Moles to Grams Example Problem Sometimes you re given a value in moles and need to convert it to grams To do this first calculate the molar mass of a sample
May 10 2021 0183 32 chemistNATE 261K subscribers Subscribed 490 81K views 2 years ago To convert a molar mass to moles you HAVE to know the amount of substance given in Grams This is because molar mass Jan 18 2024 0183 32 We can calculate it in a simple way by dividing the mass of the substance m by its amount in moles n m n The SI unit of molar mass is kg mol but the g mol unit is more commonly used Molar mass vs molecular weight It may seem as though the two quantities mean the same thing but this is not true
May 10 2023 0183 32 How to Convert Mass into Moles Example 1 Simple Compound Example 2 Complex Compound Example 3 Finding the Mass of a Number of Molecules Conclusion Avogadro s Number and the Mole Avogadro s number is a fundamental constant that represents the number of particles atoms molecules ions in one mole of