Limiting And Excess Reactants Chemistry Worksheet The reactant that is not in excess is known as the limiting reactant also known as the limiting reagent The limiting reactant is so called as it limits the amount of product that can be formed The amount of product formed will be directly proportional to the amount of limiting reactant used
Method 1 For the first method we ll determine the limiting reactant by comparing the mole ratio between Al and Cl A 2 in the balanced equation to the mole ratio actually present In this case the mole ratio of Al and Cl A 2 required by balanced equation is moles of Al moles of Cl 2 required 2 3 0 6 6 and the actual mole ratio is 1 BALANCE the equation first FeCl3 O2 Fe2O3 Cl2 a How many moles of chlorine gas can be produced if 4 moles of FeCl3 react with 4 moles of O2 SHOW ALL WORK b What is the limiting reactant c What is the excess reactant 2 Use the following BALANCED equation 2 C2H6 7 O2 4 CO2 6 H2O a
Limiting And Excess Reactants Chemistry Worksheet
 Limiting And Excess Reactants Chemistry Worksheet
Limiting And Excess Reactants Chemistry Worksheet
https://i.pinimg.com/originals/0a/e9/47/0ae947ca07e774f3b79bd1a7b23dc103.jpg
How to Find Limiting Reactant Quick amp Easy Examples Practice Problems Practice Questions
Pre-crafted templates use a time-saving solution for creating a diverse range of documents and files. These pre-designed formats and layouts can be utilized for different individual and professional tasks, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, improving the content development procedure.
Limiting And Excess Reactants Chemistry Worksheet

How To Identify The Limiting And Excess Reactants YouTube

Limiting Reagent Worksheet 2 Worksheet

Limiting Reagent Worksheet 2

Limiting Reactant Excess Reactant Percent Yield And Percent Purity

How To Determine Limiting And Excess Reactants Using Theoretical Yield

Lesson 5 Limiting Reactants And Excess Reactants YouTube

https://chem.libretexts.org/Courses/Anoka-Ramsey
This illustration shows a reaction in which hydrogen is present in excess and chlorine is the limiting reactant An alternative approach to identifying the limiting reactant involves comparing the amount of product expected for the complete reaction of each reactant

https://chem.libretexts.org/Ancillary_Materials
a determine the limiting reagent b determine the number of moles of H 2O produced c determine the number of grams of CaSO 4 produced d determine the number of grams of excess reagent left 1 make sure the equation is balanced This equation is already balanced 2 then determine the moles of each compound that you have
/simple-experiment-58b5b3325f9b586046bbfa7f.jpg?w=186)
https://www.khanacademy.org/science/chemistry/
Limiting reactant and reaction yields Worked example Calculating the amount of product formed from a limiting reactant Introduction to gravimetric analysis Volatilization gravimetry Gravimetric analysis and precipitation gravimetry 2015 AP Chemistry free response 2a part 1 of 2 2015 AP Chemistry free response 2a part 2 2 and b
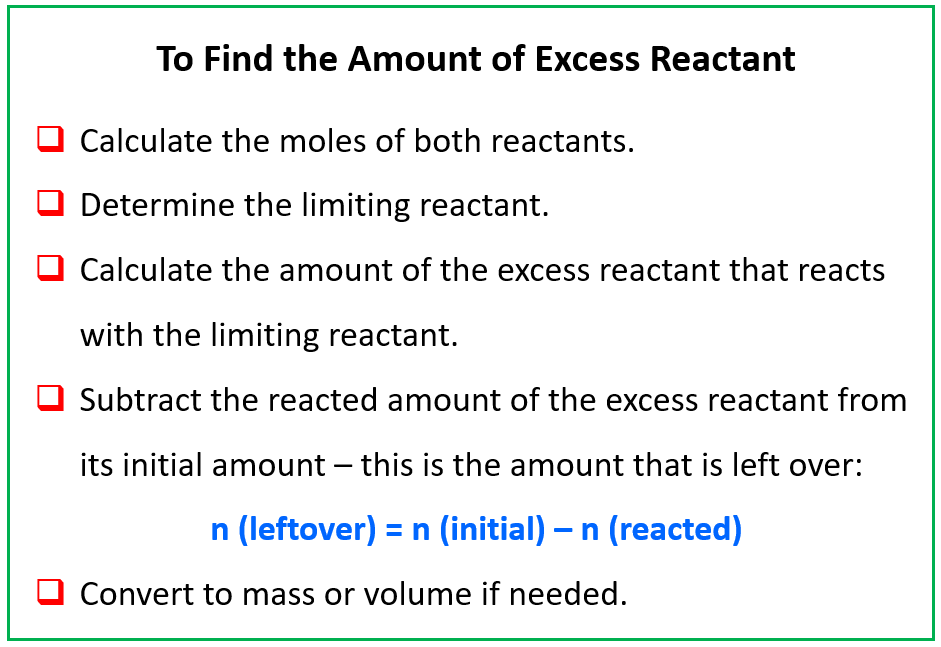
http://www.simplychemistry.org/chemistry/Pearson Chemistry
Limiting and Excess Reagents How is the amount of product in a reaction affected by an insufficient quantity of any of the reactants Many cooks follow a recipe when making a new dish They know that suffi cient quantities of all the ingredients must be available in order to follow the Key Questions How is the amount of recipe

https://chem.libretexts.org/Bookshelves/Inorganic
Jun 30 2023 0183 32 In a chemical reaction reactants that are not used up when the reaction is finished are called excess reagents The reagent that is completely used up or reacted is called the limiting reagent because its quantity limits the amount of products formed
1 Say you take a reactant A and calculate the amount of moles of another reactant B required to use up all of A How do you know which of two reactants is the limiting one You compare the Jun 2 2020 0183 32 Limiting Reactants in Solutions The concept of limiting reactants applies to reactions carried out in solution as well as to reactions involving pure substances If all the reactants but one are present in excess then the amount of the limiting reactant may be calculated as illustrated in Example PageIndex 2
Apr 10 2022 0183 32 Powerpoint lesson introducing the concept of limiting reactants and excess of a chemical reactant Contains complete lesson powerpoint with worked examples on limiting reactants and worksheets to practice more tricky examples from this higher tier chemistry only section