Mole Conversions Practice Worksheet Answers One mole of H 2 O and one mole of C O are taken in a 10 litre vessel and heated to 725 K At equilibrium 40 percent of water by mass reacts with carbon monoxide according to the
A solution of glucose in water is labelled as 10 w w what would be the molality and mole fraction of each component in the solution If the density of solution is 1 2 g mL 1 then what Click here point up 2 to get an answer to your question writing hand the reaction is fe2 so43 bacl2 baso4 fecl3how many mole of bacl2
Mole Conversions Practice Worksheet Answers
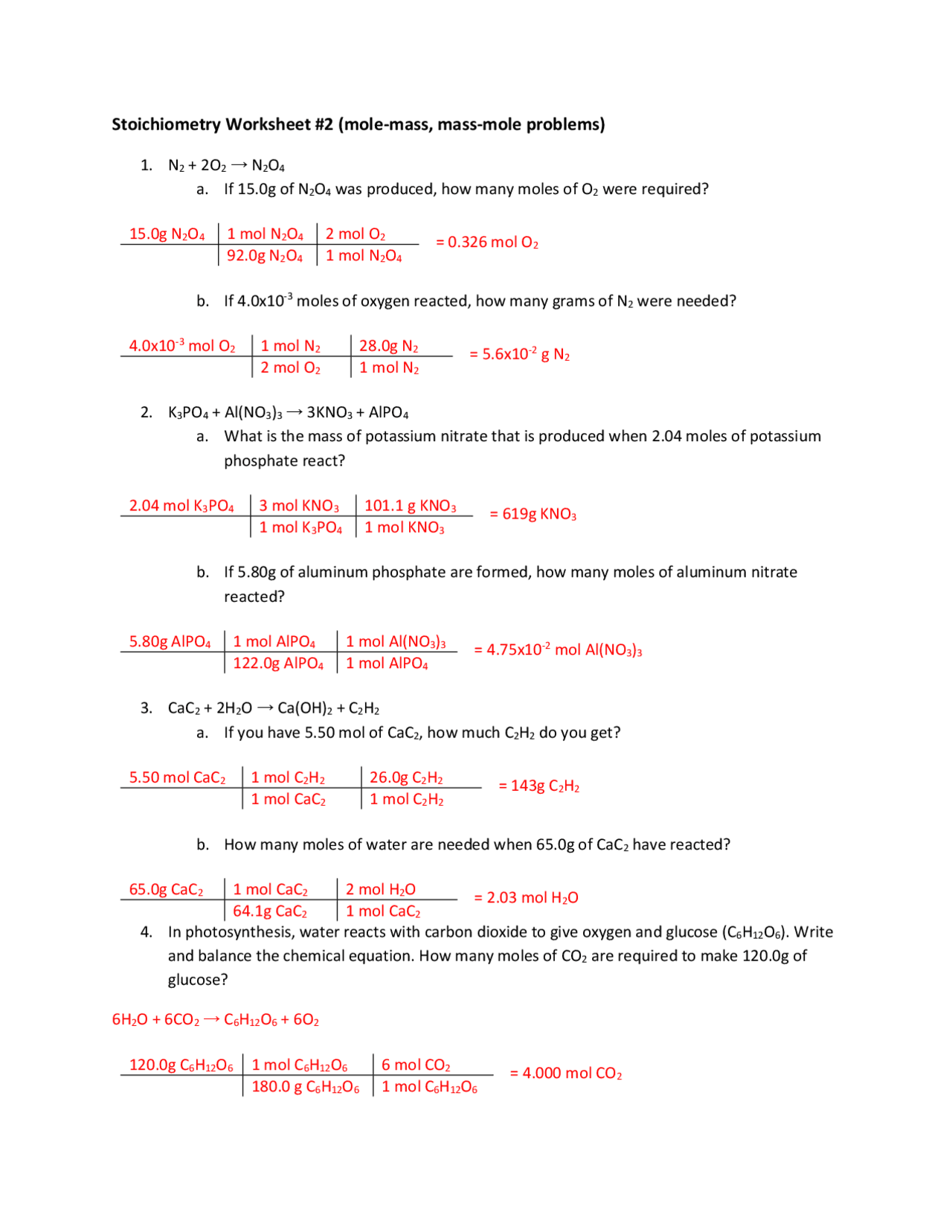 Mole Conversions Practice Worksheet Answers
Mole Conversions Practice Worksheet Answers
https://static.docsity.com/documents_first_pages/2021/04/20/574b4a1940fa840797aa40b8f41f7b44.png
Assume that each mole of TNT liberates 3 4 mathrm MJ of energy on exploding The molecular mass of TNT is 0 227 mathrm kg mathrm mol c For the same mass of
Pre-crafted templates provide a time-saving service for creating a varied variety of documents and files. These pre-designed formats and layouts can be utilized for numerous personal and professional tasks, including resumes, invitations, flyers, newsletters, reports, discussions, and more, streamlining the material development process.
Mole Conversions Practice Worksheet Answers
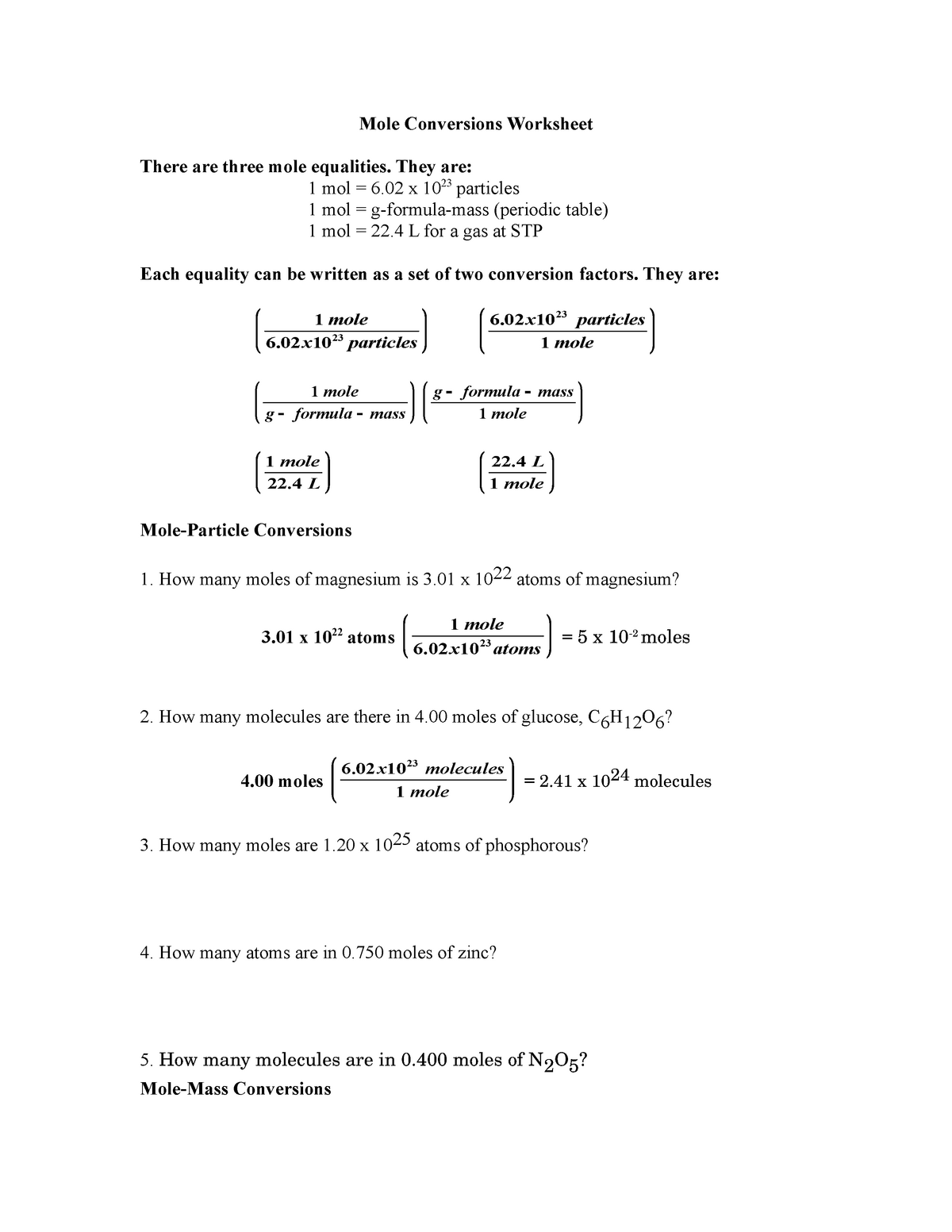
Mole Conversions Worksheet answers Mole Conversions Worksheet There

Molar Conversion Worksheet Answers Zipworksheet

Grams To Moles Conversion Worksheets

Gram To Mole Conversion Worksheet

Unit 7 Stoichiometry Mole Conversion Worksheets
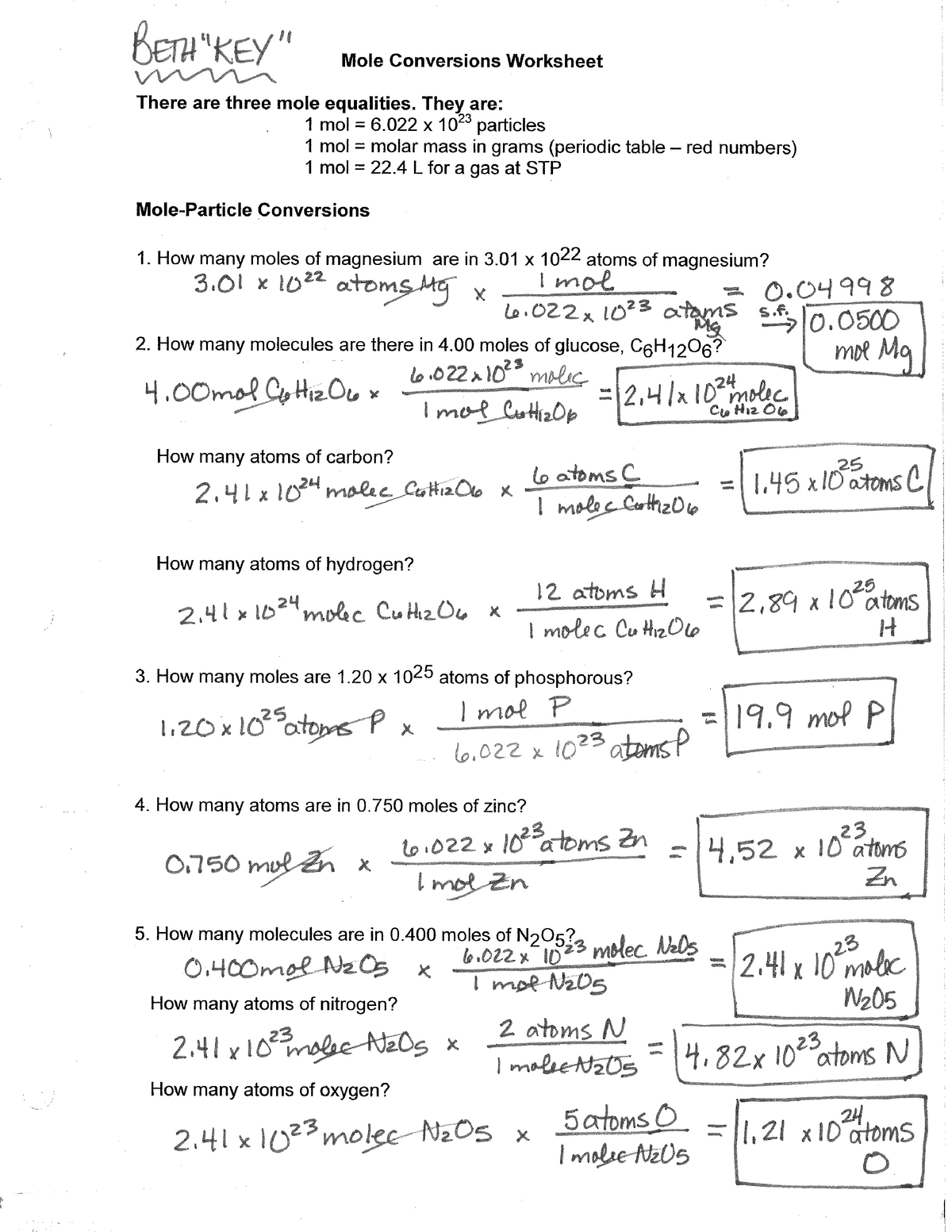
Mole Conversion Worksheet Answers CHEM 227 TAMU Studocu

https://www.toppr.com › ask › question › write-the-formula-to-calculate-t…
What is mole and what is the formula to calculate molecule in the substance View Solution Q5

https://www.toppr.com › ask › question › define-mole-fraction
Define mole fraction A solution of sucrose in water is labelled as 20 w w What would be the mole fraction of each component in the solution

https://www.toppr.com › › some-basic-concepts-of-chemistry › mole-c…
Learn the concepts of Class 11 Chemistry Some Basic Concepts of Chemistry with Videos and Stories
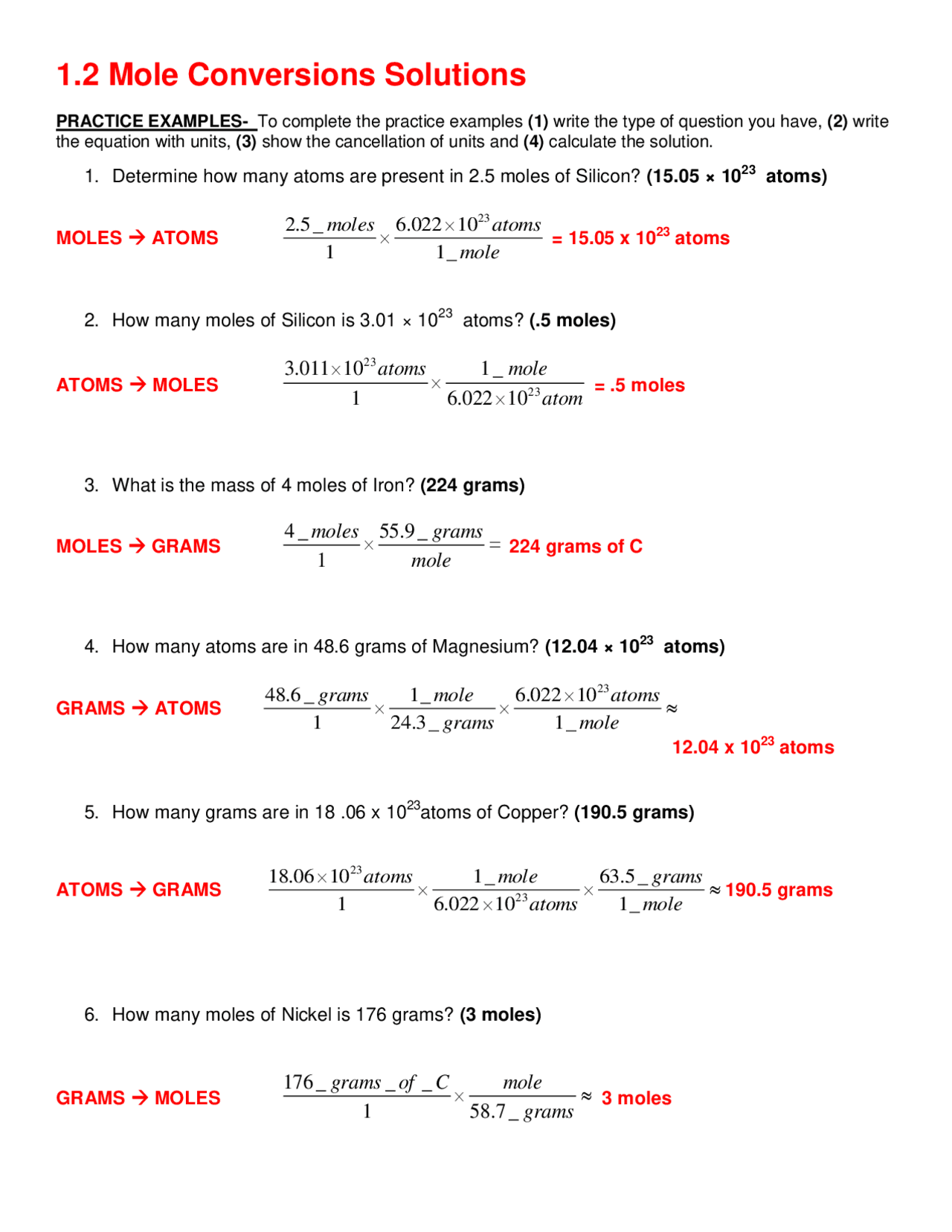
https://www.toppr.com › ask › question
The vapour pressure of a pure liquid at 25 176 C is 100 mm Hg Calculate the relative lowering vapour pressure if the mole fraction of solvent in solution is 0 8 p 186 PS Xsolute 100 15 4802 Loo

https://www.toppr.com › ask › en-us › question
One mole of C O 2 two moles of O 2 215 6 02 215 10 23 12 04 215 10 23 atoms of oxygen One mole of C O 2 6 02 215 10 23 molecules of C O 2 Note 6 02 215 10 23 is Avogadro s number and
[desc-11] [desc-12]
[desc-13]