Solubility Rules Solubility is the ability of something to dissolve in a liquid When something dissolves the tiny particles in that solute are broken down by the surrounding liquid particles
Solubility Rules Rules of solubility exist for many common ionic compounds 1 Most silver salts are insoluble except for eq AgNO 3 eq and eq Ag C 2H 3O 2 eq 2 Transition metal Using solubility rules predict the solubility in water of the following ionic compounds a PbS b AgNO 3 c Na 2CO 3 d CaI 2 Using the solubility rules predict the solubility of silver
Solubility Rules
 Solubility Rules
Solubility Rules
http://sayrechem.weebly.com/uploads/1/4/8/4/14846488/602459221_orig.png
Nov 21 2023 0183 32 The Hume Rothery rules are a great way to predict if two elements will form a solid solution These rules state that to be able to form a solid solution the two elements must have
Templates are pre-designed files or files that can be utilized for numerous purposes. They can save effort and time by offering a ready-made format and design for creating various kinds of content. Templates can be utilized for personal or professional projects, such as resumes, invites, flyers, newsletters, reports, discussions, and more.
Solubility Rules

Solubility Rules
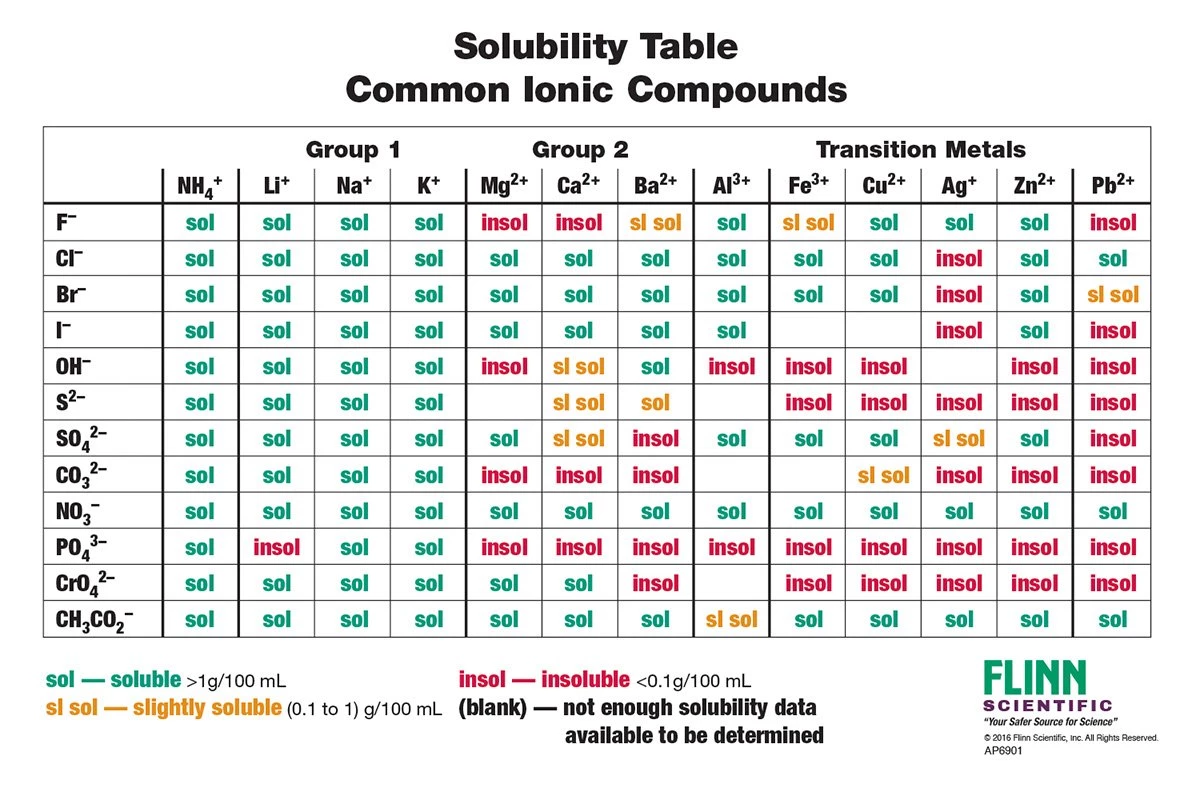
Solubility Rules
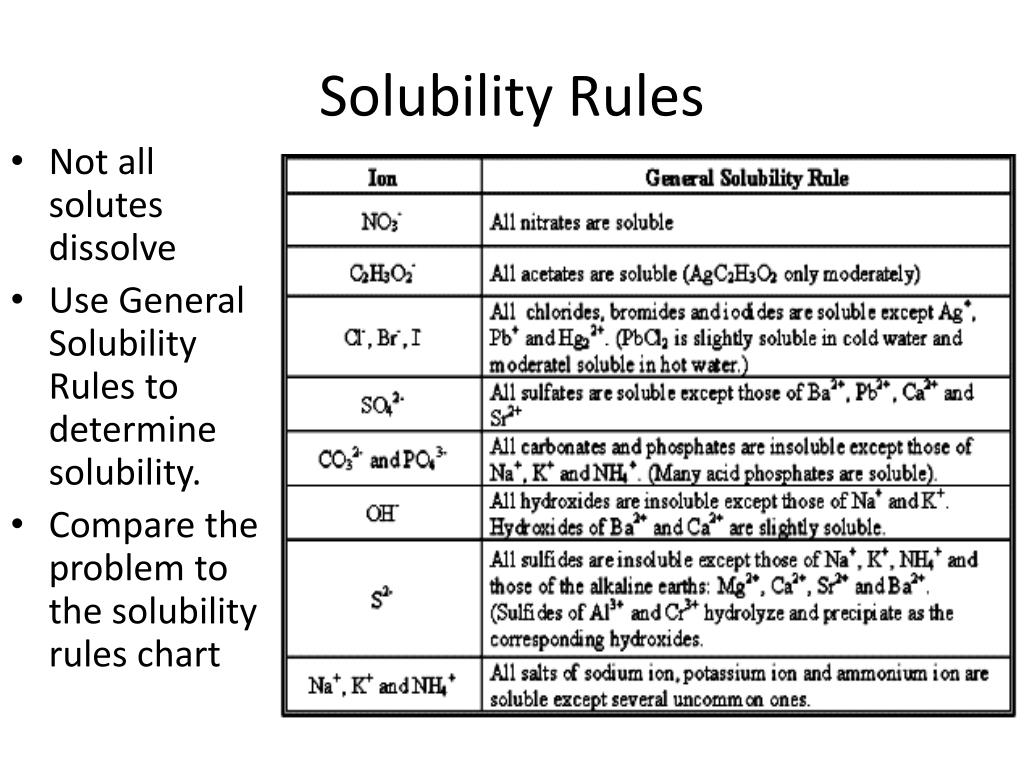
Solubility Rules

Qualitative Solubility Rules Predicting Precipitates Expii
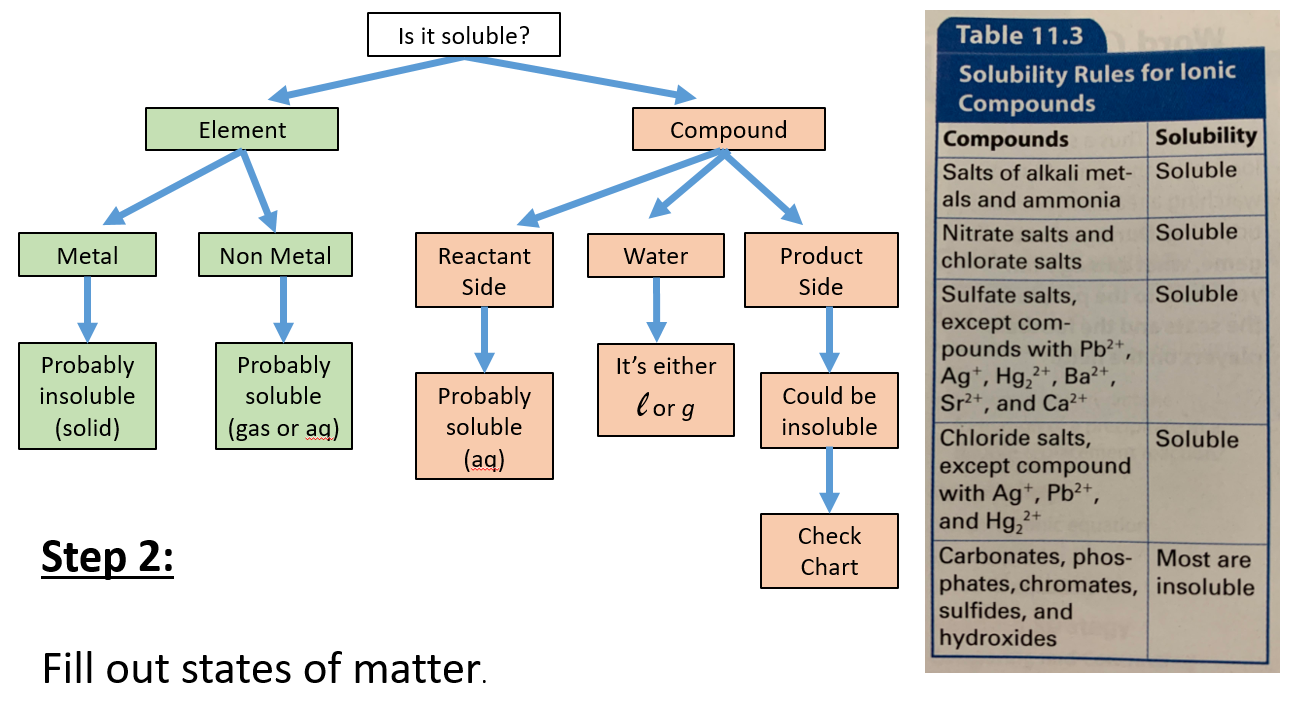
Solubility Rules Flowchart

Solubility Rules Periodic Table

https://study.com › academy › lesson › solubility-in-chemistry-definition-p…
Nov 21 2023 0183 32 The solubility definition in chemistry is the maximum amount of solute solid that gets dissolved in a given quantity of a solvent at a specific temperature
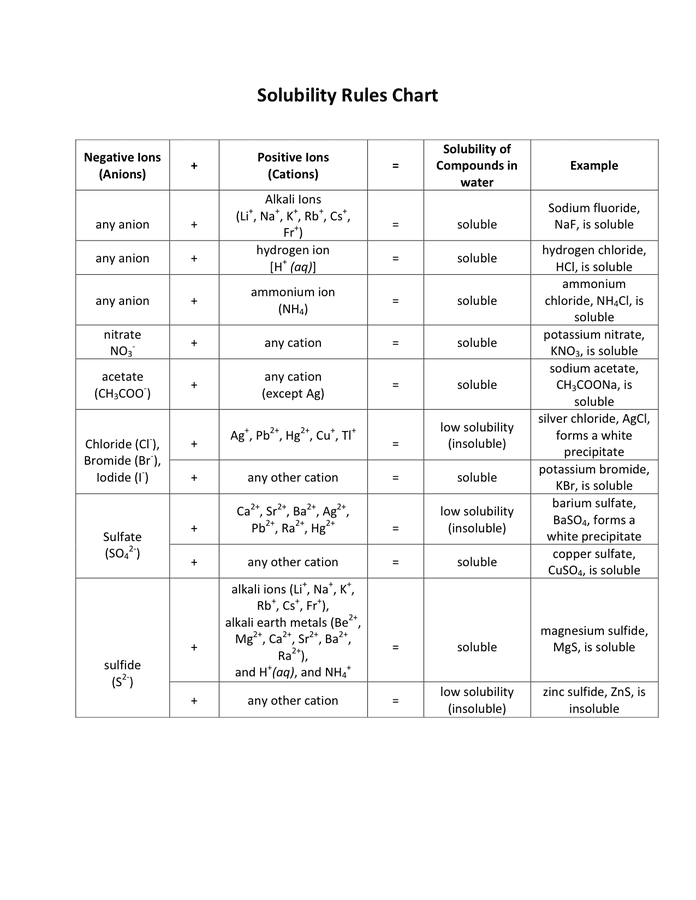
https://homework.study.com › explanation › outline-the-solubility-rules-fo…
Solubility Rules Solubility rules are a set of guidelines that state what ions are soluble slightly soluble or insoluble in an aqueous medium These rules are very helpful in determining

https://www.bartleby.com › questions-and-answers › use-the-solubility-r…
Using the solubility rules predict the solubility in water of the following ionic compounds by indicating if the compound is soluble or non soluble 1 Al OH 3 2 NH4Cl 3 PbCl2 4 KOH 5 5

https://www.bartleby.com › questions-and-answers › on-the-basis-of-the …
An aqueous sample is known to contain either Ag or Mg2 ions Treatment of the sample with NaOH produces a precipitate but treatment with KBr does not Use the solubility rules see

https://study.com › learn › lesson › solubility-common-salts-dissolve-wat…
Nov 21 2023 0183 32 Solubility Rules Now there are some pretty neat rules we can use to predict solubility For this lesson we are going to simply say that something is soluble or insoluble As
[desc-11] [desc-12]
[desc-13]