Unit 3 Worksheet 4 Chemistry Answers Nov 7 2015 0183 32 Unit 3 Worksheet 4 Quantitative Energy Problems Unit 3 Worksheet 4 Quantitative Energy Problems Keep a reasonable number of sig figsin your answers 1 How much energy must be absorbed by a 150 g sample of ice at 0 0 C that meltsand then warms to 25 0 C 2 Suppose in the Icy Hot lab that the burner transfers 325 kJ of energy to
Dec 19 2019 0183 32 Keep a reasonable number of sig figs in your answers 1 How much energy must be absorbed by a 150 g sample of ice at 0 0 C that melts and then warms to 25 0 C 2 Suppose in the Icy Hot lab that the burner transfers 325 kJ of energy to 450 g of liquid water at 20 C What mass of the water would be boiled away 3 G Unit 3 This Research investigation has been included for you to practise the skills required for the Unit 4 a ynthesis Carbon dioxide is consumed through photosynthesis and then re released to the atmosphere through respiration in plants an
Unit 3 Worksheet 4 Chemistry Answers
 Unit 3 Worksheet 4 Chemistry Answers
Unit 3 Worksheet 4 Chemistry Answers
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/b0670e1ff764791ff4fa106e17f153e7/thumb_1200_1553.png
Unit 3 ws 4 Free download as PDF File pdf or read online for free
Pre-crafted templates use a time-saving service for producing a varied variety of files and files. These pre-designed formats and layouts can be used for various individual and expert jobs, including resumes, invitations, flyers, newsletters, reports, presentations, and more, enhancing the content development procedure.
Unit 3 Worksheet 4 Chemistry Answers

Exam PROJECT2 UNIT 3 Worksheet

10 Best Images Of Chemistry Unit 8 Worksheet 4 Polar Bonds And
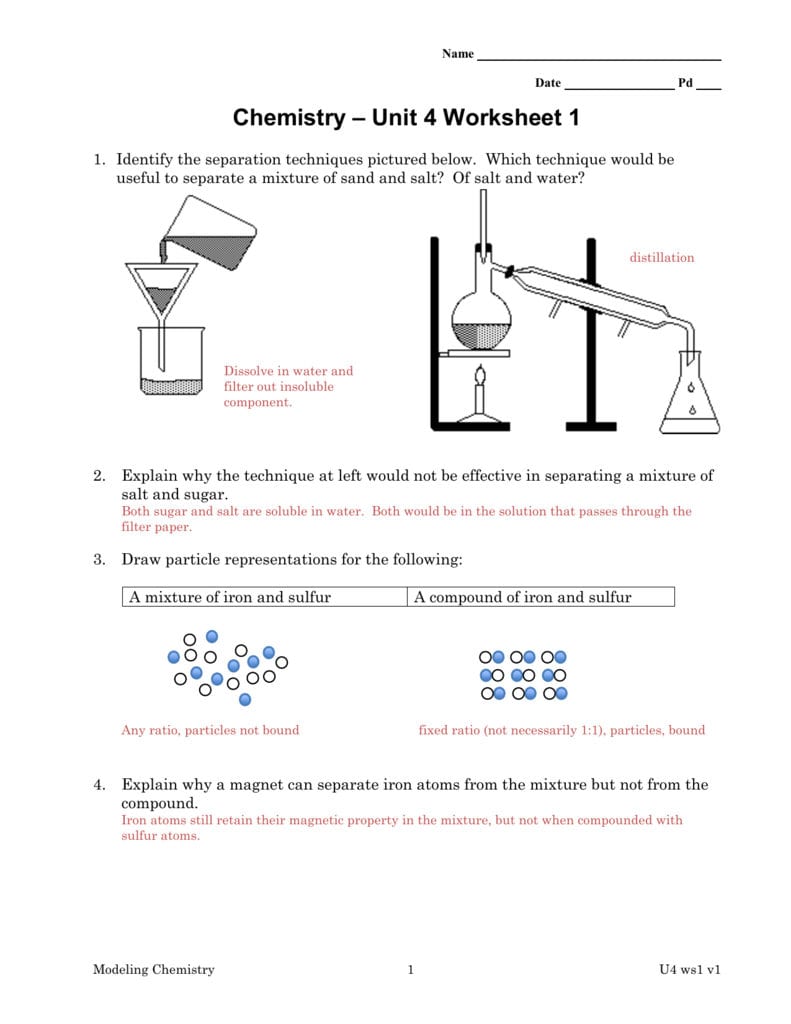
Chemistry Unit 4 Worksheet 1 Db excel

Solutions Pre Intermediate Progress Test Unit 3 Worksheet Simple Past

Unit 3 Worksheet 1 Chemistry Answer Key
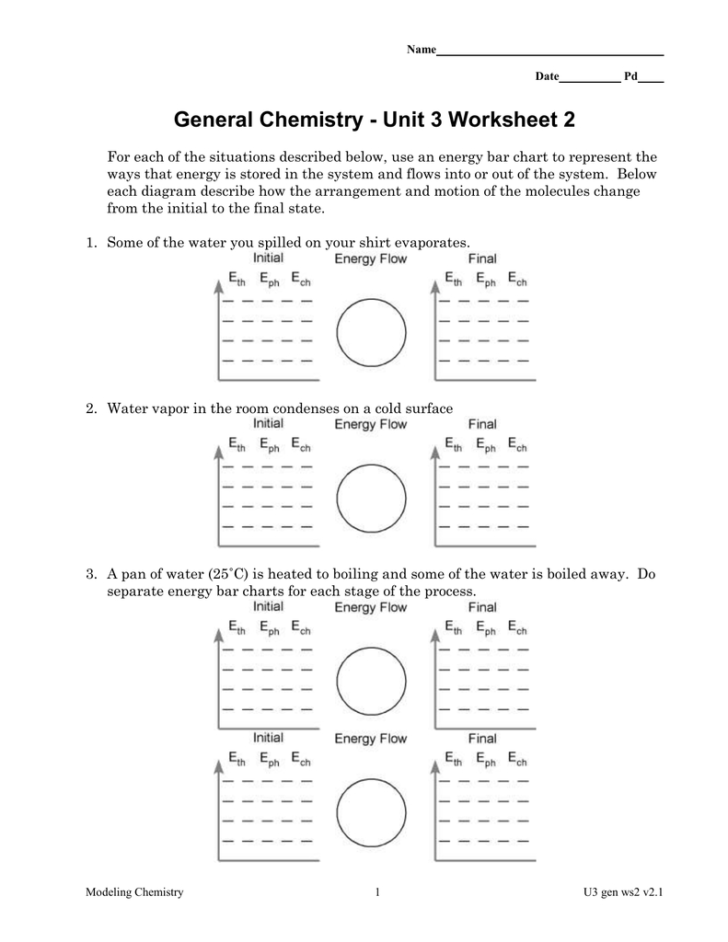
Unit 3 Worksheet 2 Chemistry Answers Db excel
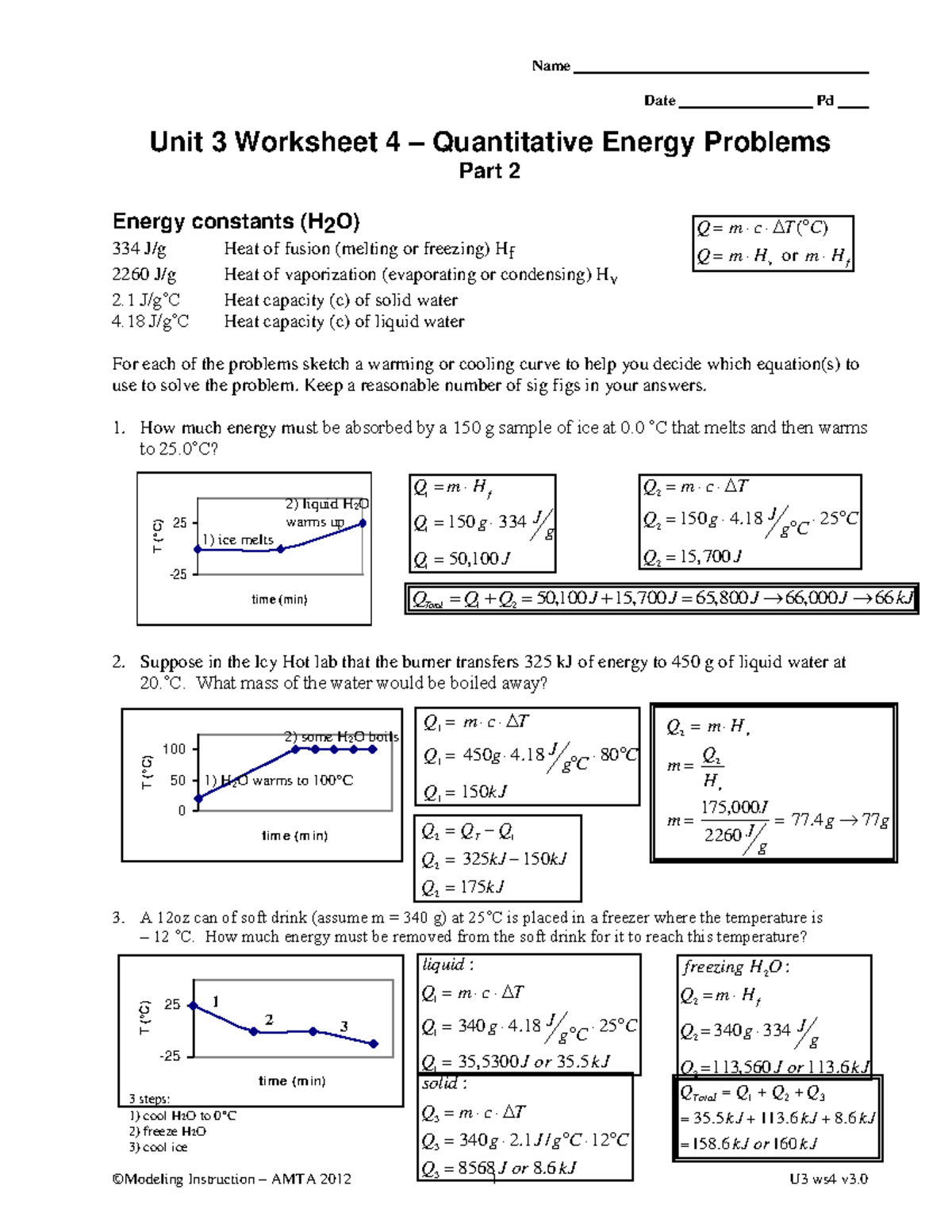
http://luckyscience.com › H-Chem Modeling
For each of the problems sketch a warming or cooling curve to help you decide which equation s to use to solve the problem Keep a reasonable number of sig figs in your answers 1 How much energy must be absorbed by a 150 g sample of ice at 0 0 C

https://www.studocu.com › en-us › document › california
Chemistry problems on enthalpy relating to topics of heat and energy Please sign in or register to post comments 169 Modeling Instruction AMTA 2012 1 U3 ws4 v3 For each of the problems sketch a warming or cooling curve to help you decide which equation s to use to solve the problem Keep a reasonable number of sig figs in your answers

https://studylib.net › doc
General Chemistry worksheet on quantitative energy problems Practice heat of fusion vaporization and heat capacity calculations High school level

https://www.wolfinchem.com › uploads
Regents Chemistry Unit 3 Heat Energy 4 1 The same amount of heat Q is added to both blocks Which block will end up at the higher temperature If you wanted to raise the temperature of 1g of water 1 176 C you would have to add 4 18J of heat energy Q So we can develop a constant for water where 4 18J per 1 gram causes a 1 176 C temperature

https://www.coursehero.com › file
Apr 11 2018 0183 32 Keep a reasonable number of sig figs in your answers 1 How much energy must be absorbed by a 150 g sample of ice at 0 0 C that melts and then warms to 25 0 C 2 Suppose in the Icy Hot lab that the burner transfers 325 kJ of energy to 450 g of liquid water at 20 0 C What mass of the water would be boiled away 3
1 B A fuel is a chemical that undergoes reaction in a way that provides useful energy This includes chemical fuels and nuclear fuels Instead a wind turbine converts the kinetic energy of wind This page provides links to answer keys for all assignments in this resource as well as a unit exam for each unit with answer key
Unit 3 Periodic Trends IE E neg 1 1 Circle the atom in each pair that has the greater ionization energy a Li or Be 2 For which of the properties does Li have a larger value than potassium Use the periodic table not the tables or charts in your text a first ionization energy b atomic radius c ionic radius d number of protons